Infective Endocarditis
Over the last three decades, the overall incidence of infective endocarditis (IE) and the associated mortality have remained constant, between 1.7 and 6.2 per 100,000 people per year, and between 10% and 30%, respectively.1–4 The clinical spectrum of IE has, however, undergone dramatic changes. These changes are noted in:
 The at-risk population
The at-risk population
 The underlying susceptible cardiac lesions
The underlying susceptible cardiac lesions
 The etiologic pathogens
The etiologic pathogens
 The clinical presentation
The clinical presentation
 The diagnostic evaluation
The diagnostic evaluation
 The antimicrobial agents
The antimicrobial agents
 The recommendations for prophylaxis
The recommendations for prophylaxis
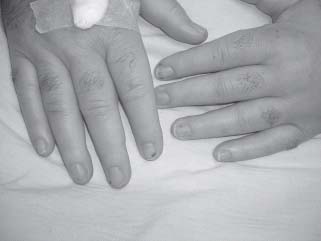
In industrialized countries, the population at risk for IE has become older, parallel to an ageing population. The attendant degenerative valve lesions such as calcific aortic stenosis and myxomatous mitral regurgitation (MR) have superseded rheumatic heart disease as the primary risk factors for IE. In developing countries where antibiotic use is not as widespread, however, rheumatic heart disease remains the key risk factor for IE. Invasive medical interventions, particularly with intravascular catheters, hemodialysis, and cardiac implantable electronic devices, have led to the emergence and prominence of health care–associated (nosocomial) IE. There has also been growth in those at risk in industrialized countries among injection drug users (IDU), and patients with prosthetic valves. The etiologic pathogens have remained largely unchanged, with viridans streptococci and staphylococci species accounting for the majority of cases. However, their relative contributions and antibiotic sensitivities have changed, mirroring the risk factors. For instance, the majority of cases in the 1960s and 1970s were caused by viridans streptococci, and 15% of cases were due to staphylococci. More recently, staphylococci have surpassed streptococci as the most common cause of IE accounting for 31% of the cases, whereas viridans streptococci were identified in 17%.2 In most urban areas, IDU may account for the majority of IE that is most commonly caused by Staphylococcus aureus.3,11 Such patients tend to be younger and present more acutely with fever and sepsis syndromes rather than as the classic Oslerian subacute and chronic presentation of fever of unknown origin, with regurgitant valvulitis, splinter hemorrhages (Fig. 35.1), Osler nodes (Fig. 35.2), Janeway lesions (Fig. 35.3), or Roth spots.
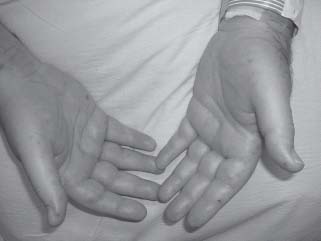
FIGURE 35.2 Osler nodes
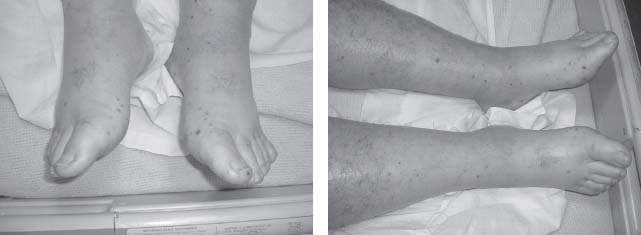
FIGURE 35.3 Janeway lesions.
The International Collaboration on Endocarditis–Prospective Cohort Study (ICE-PCS), Murdoch and coinvestigators2 prospectively collected data on 2,781 patients with definite IE by the modified Duke criteria at 58 hospitals in 25 countries from June 1, 2000, through September 1, 2005. The median age of the cohort was 57.9 years, and 72.1% had native valve endocarditis (NVE). Most patients (77%) presented early (within 30 days) with few of the classic features of IE. Recent health care exposure was found in 25% of patients. S. aureus was the most common pathogen (31.2%). Left-sided IE was more common (mitral valve 41.1%, aortic valve 37.6%). Common complications included stroke (16.9%), embolization other than stroke (22.6%), heart failure (32.3%), and intracardiac abscess (14.4%). Surgical therapy was common (48.2%), and in-hospital mortality was high (17.7%). Increased risk of in-hospital death was associated with prosthetic valves (PV), older age, pulmonary edema, S. aureus, coagulase-negative staphylococcal infection, mitral valve vegetation, and paravalvular complications. Viridans streptococcal infection and surgery were associated with a decreased risk.
DEFINITIONS
IE is defined as a microbial infection of the endocardium. Acute and subacute endocarditis are further subdivisions based on the tempo and severity of the infection1 with subacute presentation usually defined as >2 months of symptoms before diagnosis. Prosthetic valve endocarditis within 12 months after valve surgery is defined as early and is often caused by drug-resistant, surgery-related pathogens, such as methicillin-resistant Staphylococcus aureus (MRSA).1 Infections that are acquired after this period, presumably after endothelialization of the valve prosthesis, are defined as late. Late PV is more likely caused by the same set of pathogens that cause NVE, such as oral streptococci, and the HACEK group (Haemophilus species, Aggregatibacter (formerly Act-inobacillus) actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella species).1 Hospital-acquired IE has been defined as either IE with onset of symptoms >72 hours after hospitalization or IE occurring from 4 to 8 weeks after discharge from the hospital if an invasive procedure was performed during hospitalization.5
EPIDEMIOLOGY
IE is uncommon. The incidence of community-acquired NVE has remained between 1.7 and 6.2 per 100,000 person-years over the last three decades. The associated mortality has also remained unchanged (between 10% and 30%) over the same period.1–4 While three decades ago, IE was mainly a complication of rheumatic heart disease in children and young adults, in the industrialized world, IE is now mainly seen in much older adults rendered vulnerable by new risk factors including degenerative heart valve disease, valve prostheses, hemodialysis, intravascular catheters, cardiac implantable electronic devices, and IDU.1–4
Left-sided IE is more frequent than right-sided IE, accounting for over two-thirds of NVE.2,6 Prosthetic valve endocarditis of the left side has the worst prognosis, associated with mortality that can exceed 40%.5–7 The cumulative risk for PV increases with duration of the prosthesis, reported to be approximately 1% at 1 year and 2% to 3% at 5 years.8,9 Right-sided IE commonly occurs in the setting of IDU, indwelling central venous catheter, cardiac implantable electronic devices, congenital heart disease (CHD), and human immunodeficiency virus (HIV) infection.10,11 The incidence of IE associated with IDU is 150 to 2,000 per 100,000 person-years with in-hospital mortality <10%.1 Although its prognosis is generally better than that of left-sided IE, in patients with acquired immunodeficiency syndrome (AIDS), mortality of right heart endocarditis can reach 50%.12,13
Hospital-acquired IE constitutes 9% to 29% of all cases and has increased in frequency in recent years owing to greater use of invasive procedures.14,15 MRSA, coagulase-negative staphylococci, and gram-negative bacilli tend to predominate as causative agents, with mortality rates as high as 40% to 60%. Fowler et al.16 recruited 324 patients with S. aureus bacteremia caused by an infected intravascular device to define patient and bacterial characteristics associated with the development of hematogenous complications (including endocarditis). On multivariable analysis, symptom duration, hemodialysis dependence, presence of a long-term intravascular catheter, or a noncatheter device, and infection with MRSA placed the patients at a higher risk of developing hematogenous complications.
RISK FACTORS
Important risk factors for IE include:
 Degenerative valvular heart disease
Degenerative valvular heart disease
 Prosthetic heart valves
Prosthetic heart valves
 Increased exposure to nosocomial bacteremia
Increased exposure to nosocomial bacteremia
 Poor dental hygiene
Poor dental hygiene
 Long-term hemodialysis
Long-term hemodialysis
 Injection drug use
Injection drug use
 HIV infection
HIV infection
PATHOGENESIS
Four main mechanisms are responsible for the initiation and localization of infection of the endocardium:
1. A previously damaged cardiac valve or a situation in which a jet effect is produced by blood flowing from a region of high pressure to one of low pressure
2. A sterile platelet fibrin thrombus
3. A pathogen in the bloodstream
4. High titer of agglutinating antibody for the infecting organism
Damaged endocardium results in exposure of the underlying extracellular matrix proteins, which engenders thrombosis with production of tissue factor, and the deposition of fibrin and platelets.17 Pathogens then adhere to the damaged endothelium and set up infection via adhesins which include proteins and polysaccharides collectively known as Microbial Surface Component Reacting with Adhesive Matrix Molecules (MSCRAMMs).18 In experimental IE using animal models, both the magnitude of bacterial inoculum and the adhesive properties of bacteria were important in determining the likelihood of subsequent infection.19 Inoculating animals with bacterial bolus injections between 105 and 107 colony-forming units (CFU)/mL induced IE.20 Veloso et al.21 tested whether IE could also be induced by injecting the same absolute number of bacteria but at a very low level. They found that such low-grade continuous infusion (over >10 hours) was as infective as high-grade bolus infusion, confirming that perhaps the most critical factor for IE induction is total bacterial burden, rather than peak bacterial concentration. Transient, recurrent, low-grade, and short duration (1 to 100 CFU/mL for <10 minutes) bacteremia occurs during chewing and brushing teeth.22 Such “normal” cumulative exposure exceeds a single tooth extraction by the order of 105.23,24 This likely explains why most cases of IE occur without antecedent dental procedures and why health care-associated IE arises from recurrent health care–related bacteremia,25,26 a point for consideration in IE prophylaxis.
MICROBIOLOGY
In recent times, staphylococci, particularly S. aureus, have overtaken viridans streptococci as the most common cause of IE.1,2 In the future, we foresee a further shift in IE being caused by S. aureus. Coagulase-negative staphylococci are the most common pathogens in early PV1 Staphylococcus lugdunensis, a coagulase-negative organism, tends to cause a particularly virulent form of IE with high rates of perivalvular extension and metastatic seeding.1 The most common streptococci isolated from patients with IE are Streptococcus sanguis, Streptococcus bovis, Streptococcus mutans, and Streptococcus mitis.1 Endocarditis by group D streptococci, mainly Streptococcus gallolyticus, previously known as S. bovis, is prevalent among the elderly and is associated with preexisting colonic lesions.1 Polymicrobial IE is encountered most often in the setting of IDU and is uncommon.1
The HACEK group are gram-negative aerobic organisms associated with the so-called “culture-negative” IE and are now readily identified by blood culture systems. Culture-negative IE accounts for around 10% of most reported series of IE.2 The most common cause of culture-negative endocarditis is prior administration of antibiotics. To overcome this problem, special blood culture media containing charcoal have been devised to inhibit the effects of antibiotics.27 When blood cultures from patients with IE remain negative at 48 to 72 hours, the laboratory should be alerted for prolonged incubation or for plating of subcultures on enriched media. A list of organisms that cause culture-negative endocarditis is provided in Table 35.1. A comparison of microorganisms identified in published series of blood culture-negative IE shows that the most common etiologies are Bartonella species, Coxiella burnetti, and Tropheryma whipplei.28
TABLE
35.1 Organisms Causing Culture-Negative Endocarditis
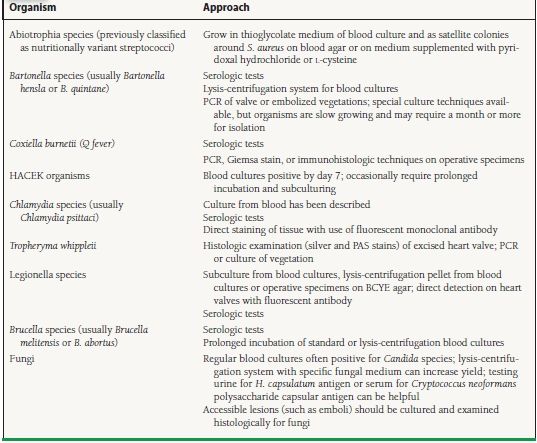
PCR, polymerase chain reaction; HACEK organisms Haemophilus species (H. parainfluenzae, H. aphrophilus, and H. paraphrophilus), Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella kingae; PAS, periodic acid–Schiff; BCYE, buffered charcoal yeast extract.
Reproduced from Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345(18):1318–1330, with permission from the Massachusetts Medical Society.
Bartonella species are fastidious gram-negative coccobacilli that are mainly transmitted by arthropod vectors. Bartonella henselae infections occur following exposure to cats or cat fleas and are associated with various diseases including cat-scratch disease, meningoencephalitis, bacillary angiomatosis, peliosis hepatitis as well as a subacute IE. Among the 24 known Bartonella species, most cases of IE are caused by B. henselae or Bartonella quintana and account for approximately 1% to 3% of IE cases and 9% to 10% of culture-negative IE.
C. burnetii is the cause of Q fever and is a common cause of IE in parts of the world where sheep, cattle, and goats are birthed.29 Coxiella tends to infect prosthetic valves or previously damaged aortic and mitral valves and causes small subendothelial vegetations that are often missed by echocardiography. The organism resides in the acidic phagolysosome, where antibiotic activity may be inhibited.
Tropheryma whipplei is a rod-shaped bacterium that is identified as PAS-positive microorganism inside the macrophages.30 It is the causative pathogen for Whipple disease characterized by chronic enteritis with malabsorption, arthritis, lymphadenopathy, uveitis, encephalitis, and dementia. It can also cause culture-negative endocarditis. Diagnosis is best achieved by polymerase chain reaction (PCR).
Brucellae are zoonotic infections.31 Humans are infected through the ingestion of contaminated meat or unpasteurized milk, the inhalation of infectious aerosols, or direct contact with infected tissues. This is mainly a disease of farmers, abattoir workers, veterinarians, and shepherds. Because vegetations are large and valve destruction commonly occurs, most patients require a combination of antimicrobial therapy and valve replacement. Legionella IE often presents as a febrile illness present over many months.32 Most patients have prosthetic valves. Embolic events are unusual with this organism. Pseudomonas aeruginosa is a rare cause of IE and occurs in the setting of IDU.2
Fungal IE is usually caused by Candida species but may also include other species such as Aspergillus and Histoplasmosis capsulatum.33 It usually manifests as bulky vegetations, perivalvular extension of infection, metastatic seeding, and embolization to large blood vessels. Predisposing factors for fungal IE are IDU, prolonged antibiotic therapy, immunosuppression, intravenous catheters, cardiac implantable electronic devices, prior or concomitant bacterial endocarditis, and disseminated fungal infection. The rate of recovery of filamentous fungi such as Aspergillus is <30% even with the lysis centrifugation system.
The Important Role of Staphlococcus aureus Bacteremia
The issue of IE in patients with prosthetic valves and S. aureus bacteremia has been recently addressed in two studies. Fowler et al.16 found that 42 of 324 (13%) patients with S. aureus bacteremia caused by intravascular device infection developed a hematogenous complication. Of these 42 patients, 31 (74%) had IE. In a prospective study by El-Ahdab et al.,34 approximately 50% of patients with prosthetic valves who developed S. aureus bacteremia had definite endocarditis. The mortality rate was high (60%) in those patients who were managed medically. The authors recommend that all such patients undergo transesophageal echocardiography (TEE) whenever possible.
CLINICAL FEATURES
IE is a systemic disease with protean manifestations. In 1885, William Osler presented the first comprehensive description of endocarditis during three Gulstonian Lectures at the Royal College of Physicians in the United Kingdom. Emanuel Libman later associated the valvular lesions with bacteremia in 1906.1 In patients presenting with classic features such as bacteremia or fungemia, active valvulitis, peripheral emboli, and immunologic vascular phenomena, the diagnosis of IE is straightforward. Fever is the most common symptom and sign; however, it may be absent in patients with congestive heart failure (CHF), severe debility, chronic renal or liver failure, previous use of antimicrobial drugs, or IE caused by less virulent organisms. Other common symptoms of subacute IE include anorexia, weight loss, malaise, and night sweats. Musculoskeletal complaints are an early symptom in subacute IE, ranging from low-back pain and myalgias to frank septic arthritis. A chronic wasting disease similar to that seen in HIV or cancer may develop in a proportion of patients with subacute IE. Pulmonary findings such as pneumonia may be the dominant feature in isolated right-sided IE. The onset of nosocomial IE is usually acute, and signs of endocarditis are infrequent.
The presentation of IE often includes extracardiac manifestations or findings that are associated with intracardiac extension of infection. Most patients with IE have a heart murmur (most commonly preexisting). A murmur or other evidence of valvular disease, especially aortic regurgitation (AR) or MR, may be a common sign in subacute IE. Patients may have petechiae on the skin, conjunctivae, or oral mucosa, as well as splenomegaly and other peripheral manifestations.
Skin lesions often provide a clue to IE diagnosis. Splinter hemorrhages (see Fig. 35.1) are 1 to 2 mm subungual brown streaks and are of greater significance when seen in the proximal nail bed rather than distal splinters that are usually related to trauma, such as that incurred while gardening. Osler nodes (see Fig. 35.2) are red, painful, indurated lesions between 2 and 15 mm, found on the palms and soles. Janeway lesions (see Fig. 35.3) are red, painless macules that appear on the palms and soles. Roth spots are boat-shaped retinal hemorrhages with a pale center. Embolization of small vegetations to the distal extremities may result in “blue toe syndrome,” which resolves in most cases without sequelae. Acute IE presents as severe sepsis with high fevers, lethargy, rapid cardiac deterioration, and shock. In acute AR, signs such as a widened pulse pressure are not usually present. In acute MR, a fourth heart sound may develop rather than the typical third heart sound seen in chronic MR. A rapid destruction of left-sided valves occurs leading to heart failure and circulatory collapse. Heart failure may result from valvular destruction, myocardial abscess, myocardial ischemia from vegetation embolization of the coronary circulation, arrhythmia such as atrial fibrillation, and sepsis.
Metastatic infection and vegetation embolization in IE can occur in virtually any organ, resulting in an acutely ischemic limb, a splenic infarct causing referred left shoulder pain from diaphragmatic irritation, a kidney infarct causing flank pain, etc. Brain complications of IE include cerebral vascular accident (CVA) from vegetation embolization or ruptured cerebral mycotic aneurysm (MA) that usually presents with intracranial hemorrhage. Anyone with a fever and a stroke should have blood cultures and be evaluated for possible IE. Symptoms and signs of cerebral involvement may be subtle however, including lethargy, confusion, and frank psychosis. Renal failure may occur from severe sepsis, embolization, immune complex deposition with acute post infectious glomerulonephritis, or may arise from therapy with antibiotics such as gentamicin. Prosthetic valve endocarditis may be manifested as an indolent illness with low-grade fever or it can be an acute febrile and toxic illness. The high frequency of invasive infection in PV results in higher rates of new or changing murmurs and of CHF. Unexplained fever in a patient with a prosthetic valve should prompt careful evaluation for PV35
DIAGNOSIS
A substantial proportion of patients with IE do not present with the classic Oslerian manifestations because of the acute nature of their illness. The diagnosis of IE requires a high index of suspicion, as well as the assimilation of clinical, laboratory, electrocardiographic, and echocardiographic data. Nonspecific laboratory abnormalities may be present, including anemia, leukocytosis, and elevated erythrocyte sedimentation rate and C-reactive protein level, and abnormal urinalysis with hematuria, proteinuria, or red cell casts. New electrocardiographic abnormalities may arise including AV or bundle-branch block, particularly in the setting of aortic-valve endocarditis with perivalvular invasion and aortic root abscess formation. Such patients need close cardiac monitoring.
The Duke Criteria
The variability of illness in IE mandates a diagnostic strategy that is both sensitive and specific. In 1981, Von Reyn et al.36 proposed very stringent Beth Israel criteria to aid in the diagnosis of IE. In 1994, a group at Duke University proposed standardized criteria for assessing patients with suspected IE.37 These criteria integrated factors predisposing patients to the development of IE, the blood-culture isolate and persistence of bacteremia, and echocardiographic findings with other clinical and laboratory information. In a review of the individual value of each component of the Duke criteria, the major microbiologic criteria had the highest relative importance. This further stresses the importance of obtaining adequate blood cultures in a patient suspected of having IE, preferably prior to the administration of antibiotics.
The Duke criteria classify patients into three categories: definite cases identified either clinically or pathologically, possible cases, and rejected cases. Numerous studies have validated the usefulness of the Duke criteria.38–43 Misclassification of culture-negative cases, the increasing role of TEE, the relative risk of endocarditis in S. aureus bacteremia, and the overly broad categorization of cases as “possible” were problems with the original criteria. A modified version of the Duke criteria has recently been proposed.44 (Table 35.2).
TABLE
35.2 Modified Duke Criteria
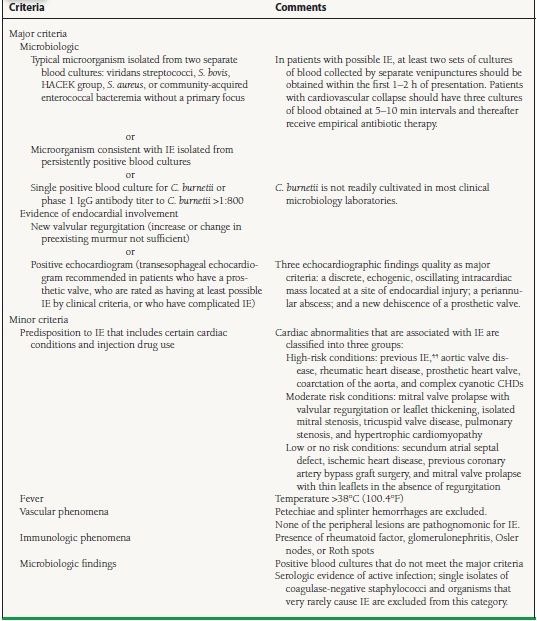
Cases are defined clinically as definite if they fulfill two major criteria, one major criterion plus three minor criteria, or five minor criteria; they are defined as possible if they fulfill one major and one minor criterion, or three minor criteria. HACEK, Haemophilus species (H. parainfluenzae, H. aphriphilus, and H. paraphrophilus), A. actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella kingae.
Reproduced from Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345(18):1318–1330, with permission from the Massachusetts Medical Society.
Blood Cultures
Blood cultures are excellent traditional tools, not only for diagnosis, but also for the determination of antibiotic susceptibility to guide therapy. According to Towns and Reller,27 best practice guidelines for blood cultures include:
 Obtaining blood cultures before starting antimicrobials whenever possible
Obtaining blood cultures before starting antimicrobials whenever possible
 Exercising strict aseptic technique and optimal skin preparation when collecting blood cultures
Exercising strict aseptic technique and optimal skin preparation when collecting blood cultures
 In acute presentations, obtaining at least two or preferably three sets of blood cultures rapidly within 5 to 10 minutes of each other prior to starting antibiotic therapy
In acute presentations, obtaining at least two or preferably three sets of blood cultures rapidly within 5 to 10 minutes of each other prior to starting antibiotic therapy
 In subacute presentations, obtaining three separate sets of blood cultures spaced 30 minutes apart
In subacute presentations, obtaining three separate sets of blood cultures spaced 30 minutes apart
 Obtaining 20 mL of blood for each sample drawn (for adults)
Obtaining 20 mL of blood for each sample drawn (for adults)