Chapter 35 Infection and Sepsis
Infection may be acquired in a hospital (nosocomial) or in the community. Most infections encountered in a cardiothoracic intensive care unit (ICU) are nosocomial; important exceptions are infective endocarditis and postpneumonic empyema. Community-acquired pneumonia is rarely managed in the cardiothoracic ICU and is not discussed here.
Nosocomial infections are reasonably common following cardiac surgery; incidences of 5.0% and 21.7% have been reported.1,2 They are associated with the development of multiple organ failure, prolonged hospital stay, and increased mortality rates.1,2 In cardiac surgery patients, the three common locations of nosocomial infection are the lungs, the central venous catheters, and the surgical site.1
PREVENTION OF NOSOCOMIAL INFECTION
The normal floras of the skin, gut, and oropharynx are responsible for most nosocomial infections.3 Risk factors for nosocomial infection include the presence of indwelling invasive devices, immunosuppression due to drugs or critical illness, hyperglycemia, inappropriate use of antimicrobials, and inadequate infection-control policies. Infection may also pass among patients and staff by direct contact (usually by the hands of healthcare workers) or, occasionally, by droplet or airborne spread. The incidence of cross-transmission of nosocomial pathogens has been reported to be 10.7 and 39.3 per 1000 patient days.4,5 General strategies for preventing nosocomial infection are discussed first; strategies for preventing specific infections are discussed in the subsequent material.
Standard Precautions
Strict adherence to a hand hygiene policy is the most effective way of preventing nosocomial infection. Hands that are dirty or are contaminated with blood or other bodily fluids should be washed with an antimicrobial soap and water. Hand decontamination may be carried out with either an alcohol-based hand rub or with an antimicrobial soap and water; this should be carried out prior to and following all patient contact. Nonsterile gloves should be worn for any intervention in which contact with bodily fluids is possible. A gown, a mask, and eye protection should be worn during any activity that is likely to generate splash or spray of bodily fluids (e.g., suctioning the trachea). When the gloves are removed, hand decontamination should be repeated.6
Patient Isolation
For protection against contact-transmitted pathogens—such as methicillin-resistant Staphylococcus aureus (MRSA)—gloves and gown should be worn. For protection against transmission of pathogens by droplet (e.g., influenza, Neisseria meningitidis) or air (e.g., tuberculosis), eye protection and a mask should be worn. In the case of airborne transmission, it should be an N-95 mask or a respirator.
Antimicrobial Prophylaxis
Antimicrobial prophylaxis reduces the incidence of surgical site infections and should be administered to all patients prior to cardiac or thoracic surgery 30 to 120 minutes prior to skin incision.7 In most circumstances a firstgeneration cephalosporin such as cephazolin is appropriate. Cephazolin is active against many staphylococci, streptococci, and some Enterobacteriaceae species (defined in Table 35-1). When infection with Haemophilus influenzae is likely (e.g., in patients with chronic obstructive pulmonary disease undergoing lung resection) a second-generation cephalosporin such as cefuroxime may be justified.
Table 35-1 Commonly Isolated Bacteria in Patients With Nosocomial Infections
| Microorganism | Notes |
|---|---|
| Gram-Positive Cocci | |
| Staphylococcus aureus (2) | Commonly present on skin and in upper respiratory tract of healthy individuals |
| Is responsible for all types of nosocomial infections, but particularly surgical site infections | |
| Coagulase-negative staphylococci (1) | Commensal of the skin |
| Weakly pathogenic but can cause CRBI and surgical site infections | |
| Common contaminant of blood cultures | |
| Enterococci (4) (e.g., E. faecalis and E. faecium) | Commensal of the gastrointestinal tract |
| Previously classified as Streptococci (group D) | |
| Responsible for CRBI and UTIs | |
| Streptococci | Commensal of the skin and upper respiratory tract |
| Cause many community-acquired infections (e.g., infective endocarditis, pneumonia, meningitis), but are relatively uncommon causes of nosocomial infection | |
| S. pneumoniae can cause nosocomial pneumonia. | |
| Gram-Negative Bacilli | |
| Enterobacteriaceae | Constitute part of the normal gut flora (i.e., enteric-gram negative bacilli) |
| The following genera cause nosocomial infection: | Responsible for most types of nosocomial infections, but particularly pneumonia and UTIs |
| Enterobacter (5) (e.g., E. cloacae) | |
| Escherichia (6) (e.g., E. coli) | |
| Klebsiella (8) (e.g., K. pneumoniae) | |
| Proteus (e.g., P. mirabilis) | |
| Serratia (e.g., S. marcescens) | |
| Morganella (e.g., M. morganii) | |
| Nonfermenting Gram-Negative Bacilli | Ubiquitous in nature |
| Pseudomonas aeruginosa (3) | Commonly colonize hospitalized patients |
| Stenotrophomonas maltophilia | Opportunistic pathogens |
| Burkholderia spp. | Rarely cause infection in healthy people |
| Responsible for most types of nosocomial infection but particularly pneumonia, wound infections, and catheter-related infection | |
| Burkholderia spp. cause infections in patients with cystic fibrosis (see Chapter 13). | |
| Gram-Negative Coccobacilli | |
| Haemophilus influenzae | Common commensal of the upper respiratory tract |
| Cause of nosocomial pneumonia | |
| Acinetobacter spp. (sometimes classified as a nonfermenting gram-negative bacillus) | Commensal on skin and in both the respiratory and gastrointestinal tracts |
| Cause of nosocomial pneumonia | |
| Moraxella catarrhalis | Commensal of the upper respiratory tract |
| Cause of nosocomial pneumonia | |
| Anaerobes (e.g., Bacteroides spp., Prevotella spp., Fusobacterium spp., Peptostreptococcus spp.) | Commensals of the upper respiratory tract and gut |
| Rarely cause pure-growth infections but may complicate polymicrobial infections, particularly those due to intraabdominal sepsis. | |
| Rarely cause pneumonia | |
| Fungi | Commensals of gastrointestinal, genitourinary, and respiratory tracts |
| Candida spp. (e.g., C. albicans (7)) | Associated with CRBI |
| Rarely cause pneumonia |
Note: The numbers in parentheses indicate the relative incidence of the pathogen as a cause of nosocomial infection. Collectively, microorganisms (1) through (8) account for 70% of all nosocomial infections.7
From Fridkin SK, Gaynes RP: Antimicrobial resistance in intensive care units. Clin Chest Med 20:303-316, 1999. CRBI, catheter-related bloodstream infection; UTI, urinary tract infection.
With the increasing prevalence of MRSA, some units favor a glycopeptide (vancomycin or teicoplanin) for prophylaxis. However, in a recent metaanalysis of randomized trials, glycopeptide use was not associated with a reduction in surgical site infections.8 Because of this, and because of concerns regarding the development of glycopeptide-resistant organisms, routine prophylaxis should remain a β-lactam.9 A glycopeptide may be used in patients who are allergic to β-lactams or when colonization with MRSA has been confirmed. The appropriate duration of treatment is controversial. The Society of Thoracic Surgeons recommends that 48 hours is effective and is unlikely to promote antimicrobial resistance.10 Single-dose regimes may also be effective but there are inconclusive data; regimes longer than 48 hours may increase the incidence of antimicrobial resistance.10 In addition, intranasal mupirocin, commenced preoperatively and continued twice daily for 5 days postoperatively, may reduce the incidence of Staphylococcus aureus infections among patients who are nasal carriers of this organism.11
Selective decontamination of the digestive tract includes the application of topical, nonabsorbable antibiotics in the oropharynx, stomach, and intestines in combination with systemic antibiotics during the first days of mechanical ventilation. It reduces the incidence of ventilator-associated pneumonia (VAP) and lowers mortality rates in selected high-risk patients (e.g., those with severe burns)12; however, it potentially increases antimicrobial resistance—especially in institutions with endemic multiresistant microorganisms—and is not widely used in cardiac surgery units.
Adjuvant Therapies
Nosocomial infection is reduced by tight glucose control (see Chapter 36), by adopting a restrictive blood transfusion policy, and by using leukoreduced blood components (see Chapter 30).
Infection Surveillance
Infection surveillance is an important method of reducing the incidence of nosocomial infection and is an essential component of quality assurance.13 It has a number of components. At its most simple, infection surveillance involves the daily assessment of each patient in the ICU for signs of infection—in particular, careful inspection of vascular access sites and surgical site wounds, daily measurement of the leukocytes, and regular measurement of body temperature. In addition, procalcitonin and C-reactive protein levels may be useful in diagnosing and prognosticating sepsis.14,15
If infection is suspected, appropriate specimens should be sent for microbiologic analysis. Specific investigations, such as transesophageal echocardiography, computed tomography (CT), or bronchoscopy, may also be indicated. Indwelling catheters should be removed as soon as they are no longer required.
SPECIFIC INFECTIONS
The microorganisms responsible for most nosocomial infections encountered in the ICU are listed in Table 35-1.
Surgical Site Infections
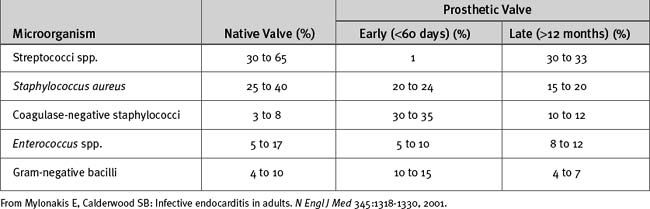
Sternal wound infections may be classified as superficial (down to the sternum) or deep (sternal osteomyelitis or mediastinitis). Surgical site infections may also occur in saphenous vein or radial artery wounds. Mediastinitis occurs in 0.5% to 2% of patients following sternotomy16 and is associated with a substantial increase in mortality rates.17 Risk factors for major infection (defined as mediastinitis, vein harvest site infection, or septicemia) following cardiac surgery are listed in Table 35-2. Mediastinitis can also be caused by perforation of the esophagus or trachea (deep mediastinitis) or by thoracic extension of neck infections.
Rights were not granted to include this table in electronic media. Please refer to the printed book.
From Fowler VG Jr, O’Brien major infections after cardiac surgery. Circulation 112:I358-I365, 2005.
Diagnosis
Surgical site infections typically present with localized cellulitis (erythema, warmth, and tenderness) and purulent discharge. With deep infections there may be sternal instability, chest pain, and systemic upset. Wound swabs and blood cultures (two sets) should be obtained before antimicrobials are given. If mediastinitis is suspected, a contrast computed tomography (CT) scan of the chest may be performed to confirm the diagnosis.18 However, most surgeons elect to proceed directly to reexploration, as it is diagnostic, provides tissue or pus for culture, and is therapeutic.
Microbiology
Staphylococci, either S. aureus or a coagulase-negative staphylococcus (mainly S. epidermidis), are responsible for the majority of postoperative sternal wound infections, with gram-negative bacilli accounting for most of the remainder.19–21 More than 50% of leg wound infections are polymicrobial; multiple enteric gram-negative organisms are commonly identified.19 Polymicrobial mediastinal infection implies a serious breach of aseptic technique, such as that which may occur during lifesaving chest reopening in the ICU. Mediastinitis due to perforation of the esophagus or trachea is also likely to be polymicrobial, commonly involving enteric gram-negative bacilli and oropharyngeal streptococci, gram-negative coccobacilli, and anaerobes.
Treatment
For superficial cellulitis, oral antimicrobial therapy for 5 to 7 days is usually sufficient. If pus is draining from the wound, the skin sutures may be removed to facilitate drainage. If there is copious pus, sternal instability, severe pain, or systemic upset, intravenous antimicrobials should be given and reexploration considered. At the completion of reexploration, the sternum is usually rewired over multiple mediastinal drains. The drains may be continuously irrigated with antimicrobial or antiseptic solution for several days.22 If there is extensive necrosis of the sternum and mediastinal tissues, closure of the sternum may be delayed. Once infection has settled and granulation tissue has formed, the wound may be closed directly or, more commonly, via a muscle flap reconstruction.23 Right ventricular laceration is a rare but usually fatal complication of open sternal wounds.24 A vacuum-assisted closure device may be used to promote the healing of open sternal (and leg) wounds and is an effective bridge between débridement and delayed definitive closure.25 Intravenous antimicrobials should be continued for at least 2 weeks; a longer course may be indicated if there are ongoing signs of infection or osteomyelitis or if a prosthetic valve is in situ. Infected leg wounds occasionally require surgical débridement and either delayed closure or closure by secondary intention (i.e., by the formation of granulation tissue).
Pleural Space Infections
Definitions and Causes
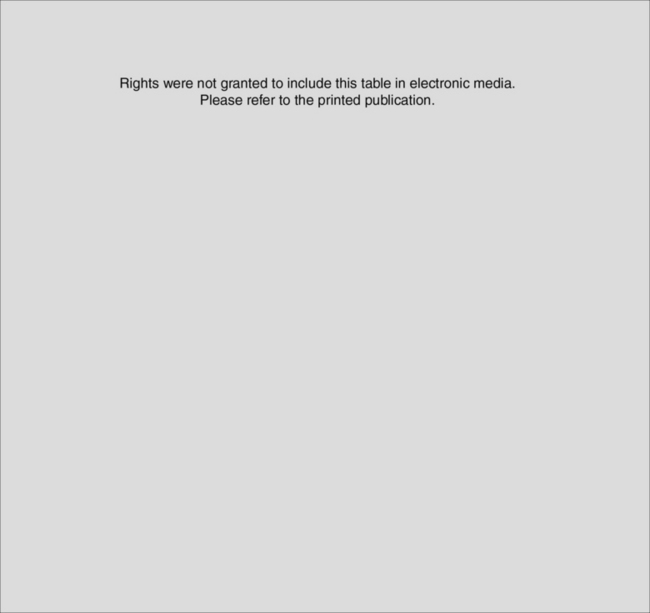
Pleural space infections include complicated exudative pleural effusions and empyema. Pleural effusions may be classified as transudative or exudative on the basis of their biochemistry (Table 35-3).26 Transudative effusions are due to disturbances in the capillary Starling forces (see Equation 1-12) caused by conditions such as heart failure and liver cirrhosis. Transudative fluid is typically clear, low in protein, and acellular. Exudative effusions are due to inflammation or infection and are associated with conditions such as bacterial pneumonia (parapneumonic effusions), tuberculosis, connective tissue disorders, lung or pleural malignancy, pulmonary embolism, and postpericardectomy syndrome (see Chapter 20). Exudative effusions may be further classified as simple or complicated (see Table 35-3). Simple exudative effusions result from increased permeability of the pleura to protein and fluid, and they do not necessarily imply infection. Complicated exudative effusions result from invasion of the pleural space by microorganisms. They are characterized by a high neutrophil count, high lactate dehydrogenase concentration (due to neutrophil lysis), and a low glucose concentration and pH (due to anaerobic glucose utilization by bacteria and neutrophils).27 The fluid is cloudy because of the presence of cellular debris. Activation of the coagulation cascade leads to fibrin deposition and the formation of loculations within the effusion. Undrained, complicated effusions can lead to empyema, which is defined as pus within the pleural space. There is marked fibroblast activity, which leads to thickening and fibrosis of the pleura.
Rights were not granted to include this table in electronic media. Please refer to the printed book.
From Maskell NA, Butland RJ: BTS guidelines for the investigation of a unilateral pleural effusion in adults. Thorax 58(suppl 2): ii8-ii17, 2003; Chapman S, Davies RJ: Recent advances in parapneumonic effusion and empyema. Curr Opin Pulmon Med 10:299-304, 2004; Light RW, MacGregor MI, Luchsinger PC, et al: Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 77: 507-513, 1972.
Diagnosis
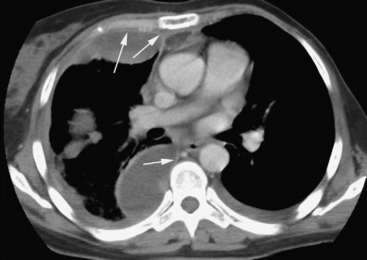
Patients with complicated effusions and empyema are typically systemically unwell and show signs of sepsis. They may have productive cough, chest pain, and respiratory distress. The appearances of chest radiographs are variable. Free-flowing effusions produce characteristic changes on erect and supine films (see Chapter 6). Loculated effusions or pleural thickening may produce nondependent pleural-based opacities (Fig. 35-1). There may be signs of the underlying cause of empyema, such as consolidation of the lung (pneumonia) or an air fluid level (bronchopleural fistula). Ultrasound scanning is useful to confirm the presence and volume of fluid in the pleural space, to identify septations, and to guide diagnostic thoracocentesis.28 Thickening and enhancement of the parietal pleura in conjunction with pleural collection as seen on contrast-enhanced CT scan are strongly suggestive of empyema (Fig. 35-2).29 CT scanning may also identify the cause. Septations are not easily seen on CT scans.
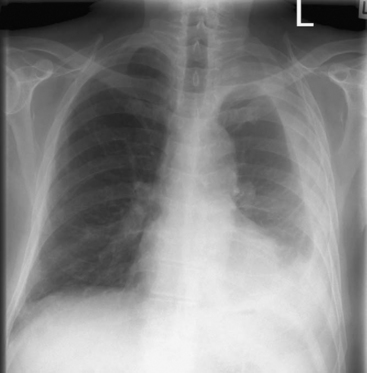
Figure 35.1 Erect chest radiograph of a left-sided nondependent pleural collection consistent with empyema.
Microbiology
Causative microorganisms include Streptococci species (S. pneumoniae, S. milleri group, viridans group); S. aureus; enteric gram-negative bacilli (particularly Klebsiella pneumoniae and Escherichia coli); Pseudomonas aeruginosa; Haemophilus influenzae; and anaerobes.30 With hospital-acquired empyema, similar microorganisms are identified, but it is more common to find Enterococcus species, MRSA, S. milleri, and multi-drug-resistant gram-negative bacilli (particularly P. aeruginosa).30 Occasionally, Candida species are responsible for empyema.
Treatment of Exudative Effusions
Simple parapneumonic effusions usually resolve spontaneously with treatment of the underlying pneumonia. In general, thoracocentesis should be performed in all parapneumonic effusions because clinical differentiation between simple and complicated effusions is unreliable.31 Effusions in patients with hypoalbuminemia, heart failure, or atelectasis are at low risk for infection and do not require sampling (but may need draining if they are large). Effusions in patients with either pneumonia or sepsis and effusions that are associated with thickening of the parietal pleura on CT scan or loculations on ultrasound scanning should be sampled.31
If thoracocentesis reveals a complicated effusion, it should be drained to prevent progression to empyema.32 If the effusion is relatively free-flowing, tube thoracostomy using a large-bore tube (e.g., 36F) is appropriate. If there are numerous septations or there has not been substantial radiographic resolution of the effusion within 24 hours of tube thoracostomy, intrapleural fibrinolytic therapy may be considered. With this technique, 100,000 IU of urokinase or 250,000 IU of streptokinase in 100 ml of 0.9% saline is instilled into the chest cavity and the tube clamped for 1 to 4 hours. This can be repeated once or twice a day for 2 to 4 days. Systemic fibrinolysis does not occur. The value of intrapleural fibrinolytic therapy, in terms of radiographic and clinical improvement, has been demonstrated in a number of small studies. However, in a recent large, randomized trial, intrapleural streptokinase was not associated with a reduced need for surgery or with improved survival rates.33 Thus, if the technique is not rapidly effective, patients should undergo surgery. Antimicrobial therapy for complicated effusions is the same as for empyema, described subsequently.
Treatment of Empyema
Once a fluid sample has been obtained by needle thoracocentesis, intravenous antimicrobials should be commenced empirically. For community-acquired empyema, amoxicillin/clavulanic acid or a secondgeneration cephalosporin (e.g., cefuroxime) combined with metronidazole may be used.34 An alternative for patients allergic to penicillins is clindamycin plus a fluoroquinolone (e.g., ciprofloxacin). For hospital-acquired empyema, a carbapenem plus vancomycin is appropriate.34 Antimicrobial therapy should be adjusted on the basis of culture and sensitivity results, but empiric cover against anaerobes should continue because these organisms are commonly present but frequently are not isolated on culture. Aminoglycosides have poor penetration into empyemas and are best avoided. Following drainage of an empyema, antimicrobial therapy should continue for a minimum of 3 weeks.34
Effective drainage of an organized empyema in which there are extensive loculations and pleural thickening requires surgery. There are two main approaches: (1) open thoracotomy and decortication; (2) video-assisted thoracoscopic débridement. The latter is a relatively new technique that appears to be safe and effective in experienced hands.35,36 During surgery, loculations must be broken down, pus drained, and any thickened visceral pleura excised (decortication) to allow the collapsed lung to reexpand. Specimens for culture should be obtained at the time of surgery and their results used to guide antimicrobial therapy. Decortication of a chronically organized empyema can result in substantial perioperative blood loss. Postoperatively there may be marked systemic inflammatory response, including fever, tachycardia, third-space fluid losses, and vasodilatation. Positive-pressure ventilation may be difficult because of air leaks, acute lung injury, and poor reexpansion of the affected lung.
Postpneumonectomy Empyema
Empyema that occurs within the first few weeks after pneumonectomy is usually caused by a bronchopleural fistula. Patients present with fever, cough, purulent or hemorrhagic sputum, respiratory distress and, occasionally, sepsis and acute respiratory failure. Chest radiographs typically demonstrate increased air content and reduced fluid levels within the pleural space. There may be soiling of the nonoperative lung demonstrated by pulmonary infiltrates on the chest radiograph (see Fig. 12-6). Empyema that occurs more than 3 months after pneumonectomy is usually due to hematogenous spread of infection into the pleural space. Patients typically present subacutely with malaise, flulike symptoms, low-grade fever, and weight loss.
The diagnosis of a bronchopleural fistula is usually confirmed by bronchoscopy or a contrast-enhanced CT scan of the chest. Alternatively, methylene blue can be instilled into the pleural space and recovered from the sputum. Needle aspiration of the thoracic space is rarely helpful in the acute setting, but if it is undertaken, it should be guided by ultrasound. Two sets of blood cultures should be obtained before starting antimicrobial therapy. Causative organisms and empiric antimicrobial therapy are as described for hospital-acquired empyema. In particular, antimicrobials against MRSA and P. aeruginosa must be administered until culture results are available. Surgical treatment of a bronchopleural fistula is described in Chapter 12.
Infective Endocarditis
Infective endocarditis37 is infection of the cardiac endothelium or of prosthetic material implanted in the heart (e.g., heart valves or pacemaker leads). It typically develops following bacteremia, for instance, after a medical procedure or injection drug use, in patients with underlying cardiac disease. Rapid diagnosis and treatment are essential because the mortality rate resulting from untreated infective endocarditis is high.
Pathophysiology and Risk Factors
The initiating event of infective endocarditis is the formation of fibrin-platelet thrombus on damaged endothelium or prosthetic material.38 With bacteremia, microorganisms seed on the thrombus. Bacterial replication and further thrombosis lead to the formation of infected vegetations (see Fig. 7-11). Clinical disease may take several weeks to develop following a bacteremic episode. Infection may result in localized tissue destruction, which can cause valve regurgitation, abscesses, or fistulae. Abscesses (see Fig. 7-14) and fistulae typically involve the aortic root. Very occasionally, large vegetations cause valve stenosis. There may be systemic emboli, including cerebral and pulmonary emboli. Pulmonary emboli arise from right-sided vegetations, whereas cerebral emboli arise from left-sided vegetations. Systemic embolization resulting from right heart lesions can occur in a patient with an intracardiac shunt or a patent foramen ovale. Right-sided lesions are associated with injection drug use. The risk for infective endocarditis with various cardiac lesions is summarized in Table 10-7; congenital cardiac lesions and rheumatic heart disease underlie most cases.
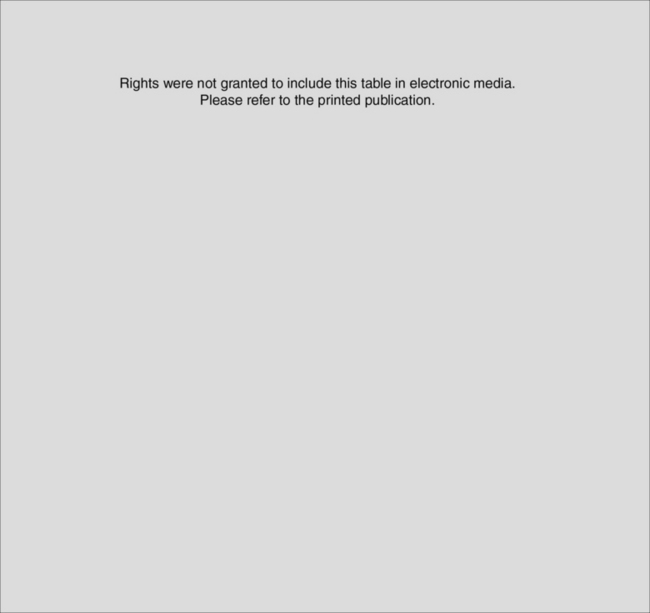
Microbiology
Gram-positive microorganisms are responsible for the majority of cases of infective endocarditis. They include (1) streptococci, particularly viridans streptococci (e.g., Streptococcus sanguis, S. mutans, S. mitis); β-hemolytic streptococci (e.g., S. pyogenes, S. agalactiae); and nonhemolytic streptococci (e.g., S. bovis); (2) staphylococci, particularly Staphylococcus aureus and coagulase-negative staphylococci (primarily S. epidermidis); (3) enterococci (e.g., Enterococcus faecium, E. faecalis). Viridans streptococci and S. aureus cause most cases of native valve endocarditis, whereas S. aureus and coagulase-negative staphylococci are important causes of prosthetic valve endocarditis occurring early after implantation (Table 35-4). Less common (<10%) causes of infective endocarditis include gram-negative bacilli, fungi, and the HACEK group of microorganisms. The HACEK group are fastidious, slow-growing, gram-negative coccobacilli from the oropharynx which, although constituting only 3% to 4% of infective endocarditis, are increasing in prevalence. They are composed of Haemophilus species, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella kingae. As much as one third of cases of infective endocarditis are culture-negative.
Diagnosis
The diagnosis of infective endocarditis rests on clinical, microbiologic, and echocardiographic criteria, as outlined in Table 35-5.37,39 Infective endocarditis should be suspected in any at-risk patient who develops a febrile illness. Three sets of blood cultures should be obtained between 30 and 60 minutes apart, and an echocardiogram should be performed (see material under Investigations). Blood samples should be incubated for at least 1 week because fastidious bacteria and fungi can occasionally be grown after the standard incubation period of 5 days.
Table 35-5 Modified Duke Criteria for Diagnosing Infective Endocarditis
| Major Criteria |
| Microbiologic |
| Typical microorganisms isolated from two separate blood cultures: viridans streptococci, Streptococcus bovis, HACEK group,* Staphylococcus aureus, or community-acquired enterococcal bacteremia, without a primary focus |
| Microorganisms consistent with infective endocarditis isolated from persistently positive blood cultures** |
| A single positive blood culture for Coxiella burnetii or phase I IgG antibody titer to C. burnetii that is greater than 1:800 |
| Evidence of endocardial involvement |
| Positive echocardiogram† (TEE recommended in patients with prosthetic valves, those rated as at least “possible endocarditis” by clinical criteria, and those with complicated endocarditis [paravalvular abscess]) |
| New valvular regurgitation |
| Minor Criteria |
| Predisposition to endocarditis by certain cardiac conditions or injection drug use |
| Fever (>38° C) |
| Vascular phenomena (conjunctival hemorrhage, Janeway lesions) |
| Immunologic phenomena (Roth spots, Osler nodes, polyarthritis, glomerulonephritis) |
| Positive microbiology not meeting major criteria |
TEE, transesophageal echocardiography.
Note: A definite diagnosis requires: (1) two major criteria; (2) one major criterion plus three minor criteria; or (3) five minor criteria. A possible diagnosis requires: (1) one major criterion and one minor criterion; (2) three minor criteria.
** The term persistently positive blood cultures is defined as at least two blood cultures drawn more than 12 hours apart, or three of three or a majority of four or more blood cultures drawn over the span of at least 1 hour.
† An echocardiogram is positive when any of the following are seen: (1) a discrete, echogenic, oscillating intracardiac mass located at the site of endocardial injury; (2) a periannular abscess; (3) a new dehiscence of a prosthetic valve.
From Mylonakis E, Calderwood SB: Infective endocarditis in adults. N Engl J Med 345:1318-1330, 2001; Li JS, Sexton DJ, Mick N, et al: Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 30:633-638, 2000.
Clinical Features
Neurologic complications occur in about one third of patients. They may take the form of a generalized encephalopathy—thought to be due to multiple microemboli—or of a focal neurologic deficit due to a macroembolus with or without cerebral abscess formation. Brain CT scanning may reveal asymptomatic cerebral abscesses. Mycotic aneurysms may involve the large arteries.
Treatment
Surgery is indicated in the following circumstances:
Risk factors for systemic embolization include vegetations greater than 10 mm diameter or highly mobile lesions attached to the anterior leaflet of the mitral valve.38 Given that more than 50% of systemic emboli involve the central nervous system, early surgery should be considered in patients with large or mobile vegetations, particularly if they are caused by S
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree