We hypothesized that the insensitivity of the electrocardiogram in identifying acute circumflex occlusion would result in differences in the distribution of the infarct-related artery (IRA) between patients with non–ST-segment elevation myocardial infarction (NSTEMI) and STEMI enrolled in the Occluded Artery Trial. We also sought to evaluate the effect of percutaneous coronary intervention to the IRA on the clinical outcomes for patients with NSTEMI. Overall, those with NSTEMI constituted 13% (n = 283) of the trial population. The circumflex IRA was overrepresented in the NSTEMI group compared to the STEMI group (42.5 vs 11.2%; p <0.0001). The 7-year clinical outcomes for the patients with NSTEMI randomized to percutaneous coronary intervention and optimal medical therapy versus optimal medical therapy alone were similar for the primary composite of death, myocardial infarction, and class IV congestive heart failure (22.3% vs 20.2%, hazard ratio 1.20, 99% confidence interval 0.60 to 2.40; p = 0.51) and the individual end points of death (13.8% vs 17.0%, hazard ratio 0.82, 99% confidence interval 0.37 to 1.84; p = 0.53), myocardial infarction (6.1 vs 5.1%, hazard ratio 1.11, 99% confidence interval 0.28 to 4.41; p = 0.84), and class IV congestive heart failure (6.7% vs 6.0%, hazard ratio 1.50, 99% confidence interval 0.37 to 6.02; p = 0.45). No interaction was seen between the electrocardiographically determined myocardial infarction type and treatment effect (p = NS). In conclusion, the occluded circumflex IRA is overrepresented in the NSTEMI population. Consistent with the overall trial results, stable patients with NSTEMI and a totally occluded IRA did not benefit from randomization to percutaneous coronary intervention.
The insensitivity of the surface electrocardiogram (ECG) to identify acute circumflex occlusion is well established. Failure or delay in recognizing acute circumflex occlusion often results in delayed or missed acute reperfusion and has been associated with a worsened outcome. We hypothesized that this insensitivity of the surface ECG would result in an overrepresentation of the culprit occluded left circumflex infarct-related artery (IRA) in patients with non–ST-segment elevation myocardial infarction (NSTEMI) enrolled in the Occluded Artery Trial (OAT). We also sought to evaluate the outcomes of subacute IRA percutaneous intervention (PCI) compared to medical therapy alone in the subgroup of patients with an electrocardiographically defined NSTEMI enrolled in the OAT.
Methods
The design, method, and results of the OAT have been previously reported. In brief, the trial randomized 2,201 stable patients with Thrombolysis In Myocardial Infarction 0-1 flow in the IRA, ≥24 hours (calendar days 3 to 28) after myocardial infarction onset to either a strategy of PCI of the IRA with optimal medical therapy (PCI group) or a strategy of optimal medical therapy alone (medical therapy). The present analysis included 35 patients recruited in 2006 as a part of a nuclear sub study, in addition to the 2,166 patients presented in the original report. The trial excluded subjects with cardiogenic shock, New York Heart Association class III-IV congestive heart failure, angina at rest or severe ischemia with stress testing, significant left main or 3-vessel coronary artery disease. By design, all patients in the present study were required to have an ejection fraction of <50% and/or culprit occlusion in a proximal coronary vessel. We compared the IRA, baseline characteristics, and outcomes for 283 subjects in the OAT with NSTEMI (absence of ST-segment elevation or Q waves or loss of R wave voltage or new left bundle branch block) to 1,918 patients with a diagnostic ECG for a recent STEMI. We also report the results of 9 years of extended follow-up (mean duration 6 years) with a combined primary end point of centrally adjudicated death, recurrent myocardial infarction, and the development of new-onset New York Heart Association class IV congestive heart failure.
A chi-square test was used to compare discrete variables and Student’s t test for continuous variables. The outcomes of death, myocardial infarction, or occurrence of class IV congestive heart failure are reported as estimates of the 7-year (84-month) event rates owing to the smaller number of events thereafter. These estimates were calculated using the Kaplan-Meier product-limit method. Patients lost to follow-up were censored at their last available contact. A covariate-adjusted hazard ratio was then quantified using a Cox proportional hazards regression model and a risk model that included demographic, clinical, and angiographic variables. We performed formal testing for interaction between presentation ECG (NSTEMI vs STEMI) and treatment effect using a Cox regression model with presentation ECG, treatment, and interaction terms all in the model. To control for the type I error rate, it was prespecified by the study protocol that p ≤0.01 would be considered as showing evidence of a difference in the secondary analyses in the OAT. SAS, version 9.2 (SAS Institute, Cary, North Carolina) was used for the statistical analyses.
Results
Overall, 283 subjects (12.9%) with NSTEMI findings were enrolled in the OAT. Of these, 136 were randomized to PCI and 147 to optimal medical therapy. Similarly, among the 1,918 OAT patients with STEMI findings, 965 were randomized to PCI and 953 to medical therapy. The subjects with NSTEMI enrolled in the OAT differed significantly from the patients with electrocardiographic evidence of a typical STEMI ( Table 1 ). By trial design, regardless of presentation ECG, angiography revealed Thrombolysis In Myocardial Infarction 0-1 flow universally in both groups.
| Parameter | NSTEMI (n = 283) | STEMI (n = 1,918) | p Value |
|---|---|---|---|
| Age (yrs) | 60.4 ± 11.7 | 58.3 ± 10.8 | 0.003 |
| Heart rate (beats/min) | 70.1 ± 11.5 | 72.0 ± 12.0 | 0.013 |
| Systolic blood pressure (mm Hg) | 123.8 ± 18.1 | 120.4 ± 17.9 | 0.002 |
| Diastolic blood pressure (mm Hg) | 72.2 ± 11.7 | 72.3 ± 11.3 | 0.88 |
| Body mass index (kg/m 2 ) | 28.8 ± 5.3 | 28.4 ± 5.0 | 0.21 |
| Estimated glomerular filtration rate (ml/min/1.73 m 2 ) | 79.5 ± 22.4 | 80.8 ± 21.4 | 0.38 |
| Men | 72.1% | 78.9% | 0.01 |
| White | 81.3% | 79.9% | 0.60 |
| Current smokers | 33.6% | 39.8% | 0.04 |
| Diabetes mellitus | 25.1% | 20.0% | 0.05 |
| Previous myocardial infarction | 20.8% | 9.8% | <0.0001 |
| Previous angina pectoris | 31.8% | 21.1% | <0.0001 |
| Hypertension | 50.5% | 48.4% | 0.50 |
| Cerebrovascular disease | 6.0% | 3.4% | 0.03 |
| Peripheral vascular disease | 4.9% | 3.6% | 0.27 |
| Renal insufficiency | 0.7% | 1.5% | 0.42 |
| Percutaneous intervention | 8.1% | 4.3% | 0.005 |
| Elevated cholesterol | 56.8% | 51.2% | 0.07 |
| Baseline heart failure factors ∗ | 30.4% | 31.9% | 0.62 |
| Ejection fraction <40% | 10.7% | 22% | <0.0001 |
| Ejection fraction (%) | 51.6 ± 10.7% | 47.1 ± 11.0% | <0.0001 |
∗ Defined as ≥1 of the following: history of heart failure before randomization, rales on examination, S3 gallop on examination, greatest Killip class >1 during index myocardial infarction before randomization, greatest New York Heart Association class >I before index myocardial infarction, or New York Heart Association class II at randomization.
The distribution of the IRA among the OAT patients according to the presentation ECG differed significantly (p <0.0001). For patients with diagnostic electrocardiographic findings consistent with STEMI, the right coronary artery (49.6%) was the most common IRA. The prevalence of left anterior descending IRA was 39.2%, and only a few of patients had a culprit occluded circumflex artery (11.2%). In contrast, the nature of the IRA differed significantly in the NSTEMI group. The left anterior descending artery was seldom the culprit (14.4%) and the predominant infarct-related vessels were the right coronary artery (43.2%) and circumflex artery (42.5%), respectively. Identification of visible collaterals to the IRA on the qualifying coronary angiogram were similar for patients with non diagnostic and diagnostic ECGs (88.9% vs 88.4%; p = 0.79). The median interval to randomization was 2 calendar days earlier for patients with a non diagnostic ECG (7 days, interquartile range 4-15, vs 9 days, interquartile range 5-17; p = 0.015).
Among the 283 patients with NSTEMI enrolled in the OAT, 136 (48.1%) were assigned to PCI, with 135 (99.3%) receiving the protocol-mandated treatment. Similarly, among the 1,918 patients with diagnostic STEMI, 965 (50.3%) were assigned to PCI, with 955 (99%) receiving the intervention. The overall rates of procedural PCI success were similar for both groups (87.5% vs 86.4%; p = 0.91). The interval from the index myocardial infarction to protocol-assigned PCI was similar for the NSTEMI and STEMI subjects (8 days, interquartile range 5–15, vs 9 days, interquartile range 5–17; p = 0.22). The interval from randomization to protocol-assigned PCI was also similar for both groups (0 to 1 day, p = 0.14).
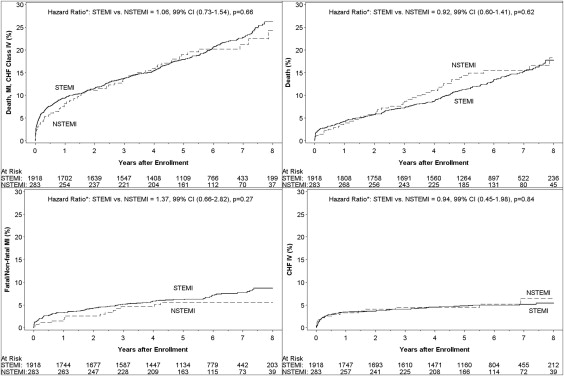
The unadjusted and adjusted 7-year (84-month) event rates and hazard ratios for the combined and individual study end points of death, myocardial infarction, and new-onset class IV congestive heart failure for patients with NSTEMI and STEMI enrolled in OAT and the Kaplan-Meier event curves are presented in Table 2 and Figure 1 , respectively. Overall, the clinical outcomes for patients with NSTEMI and STEMI were similar (p = NS for all). No interaction was found between the presentation ECG and treatment effect on the combined outcome of death, nonfatal myocardial infarction, and class IV congestive heart failure (interaction p = 0.63) or on the individual end points of death (interaction p = 0.56), fatal and nonfatal myocardial infarction (interaction p = 0.88), or class IV congestive heart failure (interaction p = 0.37).
| Outcome | NSTEMI ECG (n = 283) | Typical STEMI ECG (n = 1,918) | Unadjusted (STEMI vs NSTEMI) | Adjusted (STEMI vs NSTEMI) ∗ | Interaction p Value † | ||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 99% CI | p Value | HR | 99% CI | p Value | ||||
| Composite end point ‡ | 21.2 % (55) | 22.8% (394) | 1.06 | 0.73–1.54 | 0.66 | 1.07 | 0.73–1.56 | 0.64 | 0.63 |
| Death | 15.5% (42) | 15.3% (261) | 0.92 | 0.60–1.41 | 0.62 | 0.95 | 0.61–1.47 | 0.75 | 0.56 |
| Fatal or nonfatal myocardial infarction | 5.5 % (14) | 7.7% (128) | 1.37 | 0.66–2.82 | 0.27 | 1.45 | 0.70–3.04 | 0.19 | 0.88 |
| Class IV congestive heart failure | 6.4 % (14) | 5.1% (90) | 0.94 | 0.45–1.98 | 0.84 | 0.97 | 0.45–2.07 | 0.91 | 0.37 |
∗ Baseline risk factors for the outcomes included in multivariate analysis were age (per 10-years older), history of diabetes mellitus at study entry, lower estimated glomerular filtration rate (per 10-U decrease), lower ejection fraction at baseline (per 10 percentage points lower), history of cerebrovascular disease, history of PCI before study entry, shorter interval from qualifying MI to randomization (per day of decrease), history of peripheral vascular disease, and heart failure at baseline.
† Interaction was between type of ECG (STEMI vs NSTEMI) and treatment (PCI vs medical therapy).
‡ Death, myocardial infarction, class IV congestive heart failure.
Similarly, the unadjusted and adjusted 7-year (84-month) event rates and hazard ratio for the combined and individual study end points of death, myocardial infarction, and new-onset class IV congestive heart failure for patients with NSTEMI and STEMI, stratified by treatment assignment, are listed in Table 3 . The related Kaplan-Meier curves for NSTEMI are presented in Figure 2 . Within the NSTEMI group, the event rates for the 136 subjects assigned to PCI were similar to those of the 147 subjects assigned to medical therapy.
| Outcome | PCI | Medical Therapy | Unadjusted (PCI vs Medical Therapy) | Adjusted (PCI vs Medical Therapy) ∗ | ||||
|---|---|---|---|---|---|---|---|---|
| HR | 99% CI | p Value | HR | 99% CI | P Value | |||
| Non–ST-segment elevation myocardial infarction electrocardiogram | n = 136 | n = 147 | ||||||
| Composite end point † | 22.3% (28) | 20.2% (27) | 1.20 | 0.60–2.40 | 0.51 | 1.03 | 0.49–2.20 | 0.91 |
| Death | 13.8% (18) | 17.0% (24) | 0.82 | 0.37–1.84 | 0.53 | 0.64 | 0.26–1.55 | 0.19 |
| Fatal or nonfatal myocardial infarction | 6.1% (7) | 5.1% (7) | 1.11 | 0.28–4.41 | 0.84 | 1.03 | 0.24–4.44 | 0.96 |
| Class IV congestive heart failure | 6.7% (8) | 6.0% (6) | 1.50 | 0.37–6.02 | 0.45 | 1.51 | 0.34–6.75 | 0.47 |
| Typical ST-segment elevation myocardial infarction electrocardiogram | n = 965 | n = 953 | ||||||
| Composite end point † | 22.3% (202) | 23.3 % (192) | 1.04 | 0.81–1.35 | 0.67 | 1.04 | 0.80–1.35 | 0.73 |
| Death | 14.7% (132) | 15.9% (129) | 1.00 | 0.73–1.38 | 0.97 | 0.97 | 0.71–1.34 | 0.83 |
| Fatal or nonfatal myocardial infarction | 7.9% (70) | 7.5% (58) | 1.21 | 0.76–1.91 | 0.29 | 1.26 | 0.79–1.99 | 0.20 |
| Class IV congestive heart failure | 5.0% (43) | 5.2% (46) | 0.90 | 0.53–1.56 | 0.63 | 0.91 | 0.53–1.58 | 0.67 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


