Fig. 1
Effects of ONOO− on viability of myotubes. Myotubes were exposed to 25 μM ONOO− for 1, 2, and 6 h. Untreated myotubes served as control (CTL). Following incubation, viability of myotubes was assessed by the MTT assay. Results are means ± SE of 3 independent experiments.
3.2 ONOO− Activates p38 MAPK
p38 MAPK is a stress-activated kinase that responds to various stimuli including oxidative stress. In addition, p38 MAPK is a key mediator of catabolic signaling in skeletal muscles and was previously shown to mediate the up-regulation of the muscle-specific E3s (Rom et al. 2012b, 2013a, b; Li et al. 2005). Therefore, it was of interest to study the effects of ONOO− on p38 MAPK in skeletal myotubes. Myotubes were exposed to 25 μM ONOO− for increasing periods and activation of p38 MAPK was studied by examining the levels of phosphorylated p38 (p-p38) relative to non-phosphorylated p38. Significant phosphorylation of p38 was observed from 0.5 h until 6 h of ONOO− exposure and peak phosphorylation was evident at 1 h of exposure (Fig. 2).
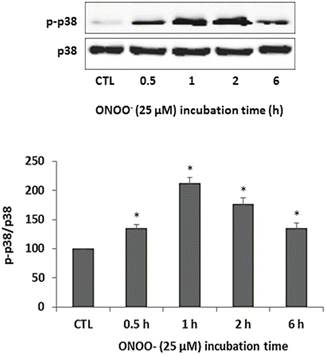

Fig. 2
ONOO − activates p38 MAPK. Myotubes were exposed to 25 μM ONOO− for 0.5, 1, 2, and 6 h. Untreated myotubes served as control (CTL). Following incubation, cell lysates were subjected to Western blot analysis with antibodies against p38 MAPK (p38) and phosphorylated p38 MAPK (p-p38). Protein levels of p-p38 and p38 were quantified by densitometry and the values of p-p38 were normalized to p38 and compared with CTL. Results are means ± SE of 3 independent experiments; *p < 0.05 vs. CTL
3.3 ONOO− Upregulates the Muscle-Specific E3s MuRF1 and Atrogin-1
ONOO− was previously shown to cause degradation of muscle proteins which was mediated by protein ubiquitination (Bar-Shai and Reznick 2006a). These findings suggest that ONOO− can activate the UPS. The muscle-specific E3s MuRF1 and atrogin-1 play a major role in the UPS by determining which muscle proteins are targeted for degradation by the proteasome (Foletta et al. 2011; Meng and Yu 2010). To study the effects of ONOO− on the expression of the above muscle-specific E3s, myotubes were exposed to 25 μM ONOO− for increasing periods and protein levels of MuRF1 and atrogin-1 were examined by Western blotting as described earlier in the Methods section. Starting from 0.5 h of exposure, ONOO− caused a significant increase in protein levels of both MuRF1 and atrogin-1. By 6 h of ONOO− exposure, protein levels of MuRF1 and atrogin-1 decreased and were not significantly different from the control (Fig. 3).
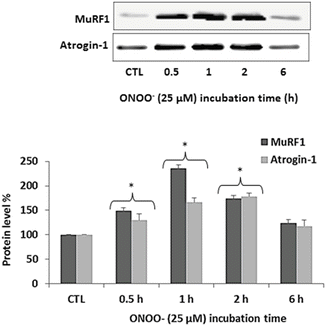

Fig. 3
ONOO − up-regulates MuRF1 and atrogin-1. Myotubes were exposed to 25 μM ONOO− for 0.5, 1, 2 and 6 h. Untreated myotubes served as control (CTL). Following incubation, cell lysates were subjected to Western blot analysis with antibodies against MuRF1 and atrogin-1. Protein levels were normalized by total protein densitometry detected by Ponceau S staining and expressed relative to the corresponding value of CTL. Results are expressed as mean ± SE of 3 independent experiments. *p < 0.05 vs. CTL
3.4 ONOO− Induces a Time-Dependent Degradation of MyHC
To study the effects of ONOO− on the main contractile muscle proteins, myotubes were exposed to 25 μM ONOO− for increasing periods and protein levels of MyHC and actin were examined by Western blotting as described earlier in the Methods section. A significant decrease in protein level of MyHC was evident from 1 h of ONOO− exposure. The level of MyHC decreased as the time of exposure to ONOO− increased (Fig. 4). No significant change in the level of actin was found.
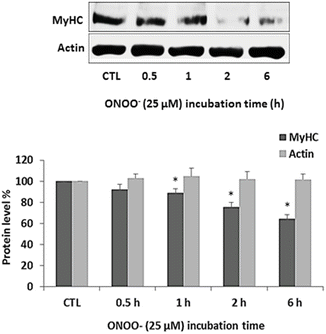

Fig. 4
ONOO− induces degradation of MyHC. Myotubes were exposed to 25 μM ONOO− for 0.5, 1, 2, and 6 h. Untreated myotubes served as control (CTL). Following incubation, cell lysates were subjected to Western blot analysis with antibodies against MyHC and actin. Protein levels were normalized to total protein and expressed relative to the corresponding value of CTL. Results are means ± SE of 3 independent experiments. *p < 0.05 vs. CTL
4 Discussion
The present study investigated the effects of ONOO− on muscle catabolism as mediated by p38 MAPK and the muscle-specific E3s atrogin-1 and MuRF1. It was found that exposure of C2 skeletal myotubes to ONOO− at the concentration of 25 μM stimulated a time-dependent degradation of MyHC that was mediated by phosphorylation of p38 MAPK and up-regulation of the muscle-specific E3s atrogin-1 and MuRF1.
It was previously shown that exposure of skeletal muscle cells to ONOO− resulted in a loss of the muscle-specific proteins MyHC and telethonin, which was mediated by a constitutive activation of NF-κB and ubiquitination of muscle proteins (Bar-Shai and Reznick 2006a, b). Moreover, exposure of rat muscle fibers to ONOO− resulted in a reduced maximum force of slow-twitch fibers and cross-linking of MyHC1 appearing as larger protein complexes (Dutka et al. 2011). Also, exposure of skeletal muscle S1-myosin ATPase (S1) to SIN-1 (3-morpholinosydnonimine), which mimics the effects of chronic exposure to ONOO−, caused inhibition, oxidation, and a partial unfolding of S1 (Tiago et al. 2006). In addition, treatment of various proteins with ONOO− resulted in enhanced proteolytic susceptibility toward degradation by the proteasome, suggesting that ONOO− can react with proteins and lead to their recognition and degradation by proteasome (Grune et al. 1998). The findings from the present study suggest that additional signaling pathways of muscle catabolism are activated in skeletal muscle in response to ONOO− exposure.
p38 MAPK is a stress-activated protein kinase that is known to be phosphorylated in response to oxidative stress. In addition, activation of p38 MAPK was demonstrated in various pro-catabolic conditions such as limb immobilization, type 2 diabetes, aging, and exposure to CS (Kaisari et al. 2013; Rom et al. 2012b, 2013a; Li et al. 2003). Therefore, it was of great interest to study the effects of ONOO− on p38 MAPK in skeletal myotubes. Indeed, a significant phosphorylation of p38 MAPK was observed shortly after exposing myotubes to ONOO− (Fig. 2). It was previously shown that exposure of C2C12 myotubes to tumor necrosis factor-alpha (TNF-α) or H2O2 resulted in up-regulation of atrogin-1, which was blunted by SB203580, a specific inhibitor of p38 MAPK. Therefore, the muscle-specific E3 atrogin-1 was suggested to be a downstream target of p38 MAPK signaling (Li et al. 2003).
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


