Modality
Sensitivity %
Specificity %
FP %/FN %
Morbidity
EBUS-FNA
93
100
1/28
Minimal
EUS-FNA
84
99.5
0.7/19
Minimal
Mediastinoscopy
78
100
0/11
Bleeding
Recurrent laryngeal nerve injury (1 %)
TEMLA
94
100
0/2.8
Recurrent laryngeal nerve injury (2.3 %)
VAMLA
94
100
0/0.9
Recurrent laryngeal nerve injury (4.6 %)
VATS
75
100
0/7
Bleeding
EBUS-FNA
EBUS–FNA has revolutionized the approach to surgical staging of the mediastinum over the last decade. Increasing familiarity with and adoption of this technique have yielded data showing reliability rivaling mediastinoscopy (considered the gold standard of pathologic pretreatment mediastinal assessment). EBUS has the advantage of being able to access most relevant mediastinal lymph nodes with minimal morbidity. EBUS is also attractive because it can be performed repeatedly without interfering with other staging techniques like mediastinoscopy. Staging EBUS should include, at the very least, examination of right and left paratracheal lymph node zones and the subcarinal lymph node station in addition to any other suspicious areas near the airway on imaging. At least three passes of each lymph node should be obtained [2, 3] and obtaining material for cell block is recommended to enhance diagnostic accuracy [4, 5].
EUS-FNA
EUS-FNA has been demonstrated to be useful to detect malignancy in select mediastinal lymph node stations such as levels 3, 7 and 8, but has limited advantage over EBUS. Some practitioners have combined EBUS and EUS (“medical mediastinoscopy”) to obtain superior results [6]. However, EUS is not used widely as a standalone modality for staging the mediastinum.
Mediastinoscopy
Mediastinoscopy was the gold standard for staging the mediastinum before the advent of EBUS. During mediastinoscopy, at the very least bilateral paratracheal zones and the subcarinal lymph node station (levels 2, 4 and 7) should be sampled [7]. An extended mediastinoscopy technique can be used to access level 5,6 lymph nodes as well. Mediastinoscopy can be used to access most mediastinal lymph node stations and is safe and accurate in experienced hands. The primary problem with mediastinoscopy has been its variable effectiveness outside expert hands. In one study, a lymph node was biopsied in only 50 % of procedures [8]. Integrating video into the mediastinoscope probably improves node acquisition and safety [9]. In addition, the procedure necessitates a general anesthetic and has the potential for serious morbidity such as bleeding catastrophes and recurrent laryngeal nerve injury (1 %). While redo mediastinoscopy has been reported to be safe in expert hands [10], in general, it is not attempted by most surgeons. Therefore, restaging after neoadjuvant therapy is problematic if this modality is used to evaluate N2 disease before therapy is initiated.
TEMLA/VAMLA
Transcervical mediastinal lymphadenectomy is an extension of mediastinoscopy in which access to the mediastinum is obtained by a larger cervical incision and a sternal lift. Of all preresectional procedures, this approaches the results of transthoracic mediastinal lymphadenectomy most closely. While proven safe in a large single center series, it has not been widely adopted [11]. Therefore, data on its reproducibility is awaited. In addition, the rate of recurrent laryngeal nerve injury is somewhat higher (2.3 %). VAMLA uses a video-mediastinoscope to perform a systematic lymph node dissection instead of a sampling as done by standard mediastinoscopy. Similar to TEMLA, the lymph node dissection approaches that done by thoracotomy or VATS, but is associated with a higher incidence of recurrent laryngeal nerve injury (4–6 %) [12]. Arguably, the accuracy of TEMLA/VAMLA exceeds open or VATS dissections because the contralateral lymph node stations are accessible through the mid-line approach. Because TEMLA and VAMLA have not been widely adopted by surgeons, their availabilities are limited. Therefore, these techniques will not be discussed further in decision making for the rest of this chapter.
VATS
Video assisted thoracic surgery has become very popular and experienced surgeons can perform the same level of lymph node dissections that were previously performed by thoracotomy. However, this approach is invasive and can only access the ipsilateral mediastinum. Therefore, its use for mediastinal staging is reserved for special situations.
Decision Making and Recommendations—Balancing Risk, Benefit and Probability
In making a decision, the clinician has to balance the risk and benefit of an action and the probability of each arm of the decision. The benefit of attempting pre-treatment pathological staging is twofold. The first is the avoidance of unnecessary surgery with its attendant mortality and morbidity in cases of occult multi-station N2 disease. The second is the potential for using neoadjuvant therapeutic strategies for single station N2 disease. This potential benefit is based on the accuracy of the various staging modalities discussed previously. Whereas accuracy of these modalities is dependent on the capabilities of local practitioners, for the purposes of this chapter, best published data will be used to make recommendations. The potential risk of pathologic staging depends on the procedure. While EBUS and EUS are very safe, mediastinoscopy and VATS carry some morbidity and even mortality. However, another “risk” is a false negative test, which is dependent on both the user and anatomic location of target nodes.
An important variable in this decision making is the probability of mediastinal disease. The most common imaging modalities used for this estimation are CT and PET-CT. CT scanning has a sensitivity and specificity of 51 and 86 % [13]. PET-CT scanning generally performs better – sensitivity and specificity of 77 and 86 %, respectively [14]. However, it is important to remember that the specificity and accuracy are based heavily on the patient population and the prevalence of inflammatory disease. In countries where this inflammatory incidence is low, performance characteristics are excellent. In other regions, specificity is lower due to other confounding mediastinal pathologies such as histoplasmosis, sarcoidosis, and tuberculosis [15].
Generally, a negative PET-CT indicates no N2 disease. This has been demonstrated in several retrospective analyses. Meyers et al. demonstrated that in clinical stage I lung cancer, the incidence of mediastinal metastasis is about 5 % [16]. A similar result was demonstrated by DeFranchi et al. from the Mayo clinic [17]. Both these studies, however, were based on clinical early stage lesions. If all lung cancer patients not having distant metastatic disease are included, the prevalence of occult (not detected by CT or PET-CT) is much higher, on the order of 10–15 % [18]. Several investigators have attempted to formulate and validate prediction systems for N2 disease with clinical criteria [19–21]. While these have the theoretical possibility of estimating the risk of mediastinal disease in an individual patient, they are based on small datasets and need significant refinement and validation before they can be recommended.
At this time broader categorizations of increased pre-test probability provide reasonable frameworks for clinical decision making. The presence of occult N2 disease is associated with the following clinical characteristics: large size (>3 cm), central tumors, adenocarcinoma or large cell carcinoma, high standardized value uptake (SUVmax) on PET-CT scan or clinical N1 disease [18, 19]. Based on the pre-test probability of mediastinal disease, the clinical situation can be classified into three categories in descending order of pre-test probability:
1.
Mediastinal disease positive on imaging
2.
Mediastinal disease negative on imaging with primary tumor characteristics denoting high risk for mediastinal disease
3.
Mediastinal disease negative on imaging with primary tumor characteristics denoting low risk for mediastinal disease
The following sections describe the rationale and approach to each of these situations and are summarized in Fig. 6.1.
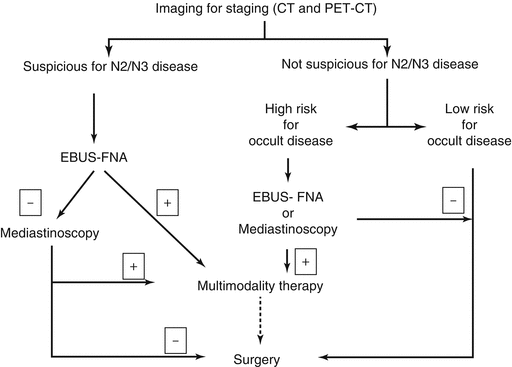

Fig. 6.1
Decision pathway for pathological examination of mediastinum in patients with non-small cell lung cancer. EBUS-FNA Endobronchial ultrasound guided fine needle aspiration, CT computerized tomography, PET-CT positron emission tomography with computerized tomography
Mediastinal Disease Positive on Imaging
The goal of pathological assessment of mediastinal lymph nodes is primarily to confirm the diagnosis of N2 or N3 disease beyond doubt so that definitive treatment recommendations may be made. This is particularly true in the case of bulky N2 lymphadenopathy, multistation N2 lymphadenopathy or N3 lymph node disease, because, in most centers, this warrants non-surgical therapy. Provided all methods of staging with the requisite expertise are available, the current method of choice for obtaining the diagnosis in this setting is EBUS-FNA. Advantages of EBUS-FNA over other modalities include the ability to stage all relevant lymph nodes stations and minimal morbidity. Another distinct advantage of EBUS-FNA in the case of single station N2 disease is the ability to restage the mediastinum after therapy with mediastinoscopy or TEMLA. While small case series demonstrating the safety of redo mediastinoscopy exist, most practitioners avoid this procedure especially if radiation was used. A negative EBUS in this situation should be followed up with mediastinoscopy before initial treatment, as a significant proportion of patients with a mediastinum suspicious on imaging and negative on EBUS will be positive on mediastinoscopy. In case EBUS-FNA is not available in a given center, the procedure of choice is mediastinoscopy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


