3 Indications for and Results of Medical Thoracoscopy/Pleuroscopy
Medical thoracoscopy/pleuroscopy (MT/P) is today primarily a diagnostic procedure, but it can also be applied for therapeutic purposes (Mathur et al. 1994; Harris et al. 1995; Medford et al. 2010). Pleural effusions are by far the leading indication for MT/P both for diagnosis, mainly in exudates of unknown etiology, and for staging in diffuse malignant mesothelioma or lung cancer, and for treatment by talc pleurodesis in malignant or other recurrent effusions (Rodriguez-Panadero et al. 2006) or in cases of empyema (Brutsche et al. 2005). Staging of spontaneous pneumothorax combined with local treatment, in stages I and II, is also an excellent indication for MT/P (Boutin et al. 1995 a). Those who are familiar with the technique can use it, for example, for biopsies from the diaphragm, the lung, the mediastinum, and the pericardium, or for sympathectomy (Tassi et al. 2006). In addition, MT/P offers a remarkable tool for research as a “gold standard” in the study of pleural effusion and pneumothorax (Loddenkemper 1998) (see Chapter 6, p. 56). A survey performed in the then West Germany in 1984 gives an impression of the indications that were used by pneumologist at that time (Fig. 3.1).
At the Lungenklinik Heckeshorn in Berlin, during the last three decades there has been a definite trend toward an increase in the application of MT/P in pleural effusions, both in absolute and in relative numbers, as shown in Figure 3.2. Correspondingly, there has been a decline in the other indications. This decrease in all indications other than pleural effusions is explained by several factors (Table 3.1). That the absolute number of MT/P in pleural effusions has risen so much is due to the fact that more and more medical thoracoscopies/pleuroscopies are performed in pleural effusions for diagnostic and therapeutic purposes. However, the absolute number of medical thoracoscopies in all indications has slightly decreased, since other hospitals have also introduced the thoracoscopic technique in the meantime and since VATS is performed in some of the other indications.
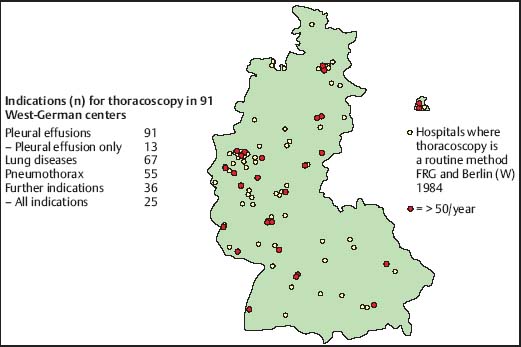
Fig. 3.1 Survey of the indications for thoracoscopy in the then West Germany (FRG) 1984. Hospitals with more than 50 thoracoscopies per year are marked in red on the map.
Table 3.1 Reasons for the change in indications for medical thoracoscopy/pleuroscopy
• Introduction of transbronchial lung biopsy, bronchoalveolar lavage and HR-CT (diffuse lung diseases) • New imaging techniques (CT, MRI) in localized lesions (lung, chest wall, diaphragm, mediastinum) • Introduction of surgical thoracoscopy (VATS) |
The indications for diagnosis in localized and chest-wall lesions have diminished considerably because imaging techniques such as computed tomography (CT) or magnetic resonance imaging (MRI) very often deliver the diagnosis or allow the differentiation between malignant and benign disease. In addition, VATS or “surgical thoracoscopy” can be performed by preference in these indications for diagnosis and for the simultaneous removal of the lesion. Furthermore, the indications for thoracoscopic/pleuroscopic lung biopsies in diffuse lung diseases have decreased. This decrease is due to the improved diagnostic results of bronchoscopy using transbronchial lung biopsies and bronchoalveolar lavage as well as to the development of high-resolution CT (HRCT), which improves the definition of structural changes (e.g., the degree of fibrosis) and sometimes even gives the diagnosis (e.g., in pulmonary Langerhans cell histiocytosis).
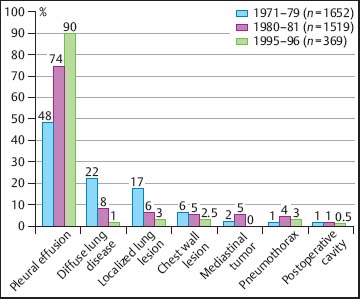
Fig. 3.2 Change in indications for medical thoracoscopy at the Lungenklinik Heckeshorn, Berlin during the last three decades of the twentieth century.
The low number of pneumothorax patients in this series is explained by the policy at Lungenklinik Heckeshorn whereby the Department of Thoracic Surgery takes care of almost all patients admitted with spontaneous pneumothorax (Kaiser 2000; Kaiser et al. 2000). However, the surgeons include the routine application of a thoracoscope in nearly all cases for the inspection of the pleural cavity, prior to the insertion of the chest tube through the cannula. This is done under local anesthesia, which actually means that they perform a “medical thoracoscopy.” The same approach is frequently used by the thoracic surgeons in cases of parapneumonic effusions and empyema, which are traditionally treated at this institution almost exclusively in the Thoracic Surgery Department (Kaiser 1989).
Management of Pleural Effusions
Besides diagnosis, patients with pleural disorders may require evacuation of pleural fluid, guided parietal pleural biopsy, lung biopsy, or pleurodesis (Lee and Colt 2005). MT/P has its main role in the invasive diagnosis of otherwise undetermined pleural effusions and in local treatment (Fig. 3.3). In the following, a general overview of the different diagnostic and therapeutic possibilities is given and, in particular, the potential role of MT/P in the management of pleural effusions of different origins is described.
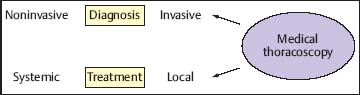
Fig. 3.3 The role of medical thoracoscopy/pleuroscopy in the management of pleural effusions: invasive diagnosis and local treatment.
Diagnostic Approach
Pleural effusions are common problems in pulmonary practice. It is estimated that approximately 25% of all consultations in the pulmonary service in the United States concern pleural diseases (Collins and Sahn 1987; Light 1997). The incidence of pleural effusions is calculated as being 300 per 100 000 in the United States (Light 2007) and 320 per 100 000 for Central Bohemia (Marel et al. 1993; Marel 2002). Pleural effusions may have many origins. They can be either the consequence of disease localized in the chest or the manifestation of extrathoracic or systemic diseases (Light 2007). The main cause (36%) is cardiac failure with unilateral or bilateral effusions. Among noncardiac effusions, parapneumonic effusions are the most common at 34%. Malignant pleural effusions follow, with 23% of cases. Pleural effusion is secondary to pulmonary embolism in 17%, to viral etiology in 11 %, to liver cirrhosis in 6%, and to gastrointestinal diseases in 3% of cases.
Many other possible causes, albeit rare or extremely rare, play an important role in differential diagnosis. The discrepancy between the estimated incidence and the frequency distribution in the respiratory literature, in which the malignant causes are the most common—followed by infectious causes and idiopathic effusions—most probably results from patient selection (Loddenkemper 2004 a).
Conversely, it may be concluded that, apart from cardiac effusions, effusions as sequelae of pneumonia, pulmonary embolism, liver cirrhosis, gastrointestinal disease, and autoimmune disease are usually easy to diagnose and, therefore, less frequently referred to the pulmonary specialist.
Thus, in the majority of cases, the etiology can be established by case history, clinical presentation, and imaging techniques. The next diagnostic step in a pleural effusion of still indeterminate origin is thoracentesis and direct examination of the pleural fluid (Fig. 3.4). Thoracentesis is indicated in all cases of pleural effusion of unknown origin, and in those effusions where the diagnosis was assumed on the basis of clinical criteria but which do not resolve under appropriate treatment (Light 2007).
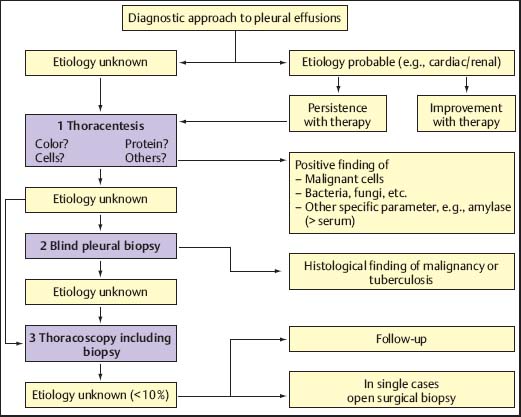
Fig. 3.4 Algorithm for the diagnostic approach to pleural effusions.
In many cases, evaluation of the pleural fluid yields valuable diagnostic information or even permits a clear diagnosis. The most important criteria are appearance (color), protein content, and cellular components. Additional laboratory values as well as microbiological tests may also be helpful. However, even after extensive diagnostic work-up of the pleural fluid, the origin of several pleural effusions remains unclear (Storey et al. 1976; Hirsch et al. 1979; Lamy et al. 1980; Ferrer et al. 1996); additional biopsy techniques may therefore be indicated, but these can provide the final diagnosis only in cases with a distinct pathology of the pleural cavity, almost exclusively in malignancy and tuberculosis (Mathur and Loddenkemper 2002). Boutin and co-workers found that after extensive work-up, out of 1000 consecutive patients with pleural effusions, MT/P was indicated in 215 with chronic effusions, in whom it established the diagnosis in up to 97% (Boutin et al. 1981a).
Blind needle biopsies of the chest-wall pleura may establish the diagnosis in a few cases, particularly in tuberculous pleurisy (Valdés et al. 1998). The additional diagnostic yield in malignant pleural effusions is usually small, and it can be debated whether blind needle biopsy should routinely be performed in cases where a strong suspicion of malignancy is given (Antony et al. 2000). The next step for obtaining pleural biopsies, which offer a high diagnostic sensitivity in malignant pleural effusions together with a very high specificity to exclude these diagnoses, is MT/P or VATS (Rodriguez-Panadero et al. 2006). The choice between these two procedures is mainly determined by the facilities and preference of the diagnosing institution. In our view, MT/P should be preferred since it can be performed under local anesthesia or conscious sedation, without the need for general anesthesia, double-lumen intubation, and single-lung ventilation (Boutin et al. 1993), and thus is less cumbersome for the patient. Since it can be performed in an endoscopy suite with fewer personnel and using nondisposable instruments, it is also less expensive. Besides its diagnostic role, MT/P is useful in certain therapeutic circumstances, in particular to prevent recurrent pleural effusion by talc poudrage (Rodriguez-Panadero et al. 2006; Froudarakis 2008). The main indication is recurrent malignant pleural effusion (Antony et al. 2000).
In their concluding chapter on future directions in their Textbook of Pleural Disease, the editors Light and Lee (2008) discuss the potential role of medical thoracoscopy in the diagnosis and treatment of pleural diseases: Gary Lee predicts that thoracoscopy will be used more frequently in the diagnosis, while Richard Light predicts that thoracoscopy will be used less frequently in the diagnosis of pleural disease, as better noninvasive diagnostic tests will be developed. Both predict that thoracoscopy will be used early in the treatment of parapneumonic effusions and Richard Light predicts that more thoracic surgeons will become proficient at thoracoscopy and chest physicians will only perform the procedure where there are no thoracic surgeons trained for and interested in doing thoracoscopy. In contrast, Gary Lee states that there is already an increasing number of chest physicians performing thoracoscopy worldwide and predicts that increasingly surgeons will only be involved in complicated cases. Richard Light believes that the demand for thoracoscopy will be relatively low (e.g., compared with bronchoscopy). As such, only a small percentage, not the majority of chest physicians will become proficient at thoracoscopy (Lee and Light 2008).
Table 3.2 Advantages of medical thoracoscopy/pleuroscopy in the diagnosis of pleural effusions
• Fast and definite biopsy diagnosis including TB culture and hormone receptor assay • Biopsies not only from chest wall pleura but also from diaphragm, lung, and mediastinum • Staging in lung cancer and diffuse mesothelioma • Exclusion of malignancy and tuberculosis with high probability • Gold standard for scientific studies |
Table 3.3 Advantages of medical thoracoscopy in the treatment of pleural effusions
• Complete and immediate fluid removal • Evaluation of loculations (TB, empyema) • Evaluation of the reexpansion potential of the lung • Talc poudrage for pleurodesis with uniform distribution of talc (6-10 mL) under visual control (=nonsurgical gold standard) • Early start to drug treatment, e.g., TB • In addition better diagnosis + staging |
In the same textbook, the surgeons David A. Waller and Antonio E. Martin-Ucar from the United Kingdom predict in their chapter on “Surgery for Pleural Diseases” that medical thoracoscopy will be increasingly applied as initial diagnostic procedure, together with pleurodesis, when considering a malignant etiology of the pleural effusion (Waller and Martin-Ucar 2008).
Some authors feel that it is not necessary to perform MT/P in persistent pleural effusions to obtain a correct diagnosis, because the disease is incurable anyway and the prognosis is therefore poor. However, Harris and colleagues found that the clinical management was influenced by thoracoscopy in 155 out of 182 (85%) patients, of whom 98 (54%) had a malignant disease (Harris et al. 1995). Thoracoscopic findings resulted in important changes in treatment. Further surgical or therapeutic procedures were performed or deferred in 133 patients (72%), and subsequent medical treatment was directly affected by thoracoscopy in 66 patients (36%), of whom 36 underwent subsequent chemotherapy and 10 underwent radiotherapy.
The current policy of most thoracoscopists is therefore to use MT/P directly in almost all pleural exudates of undetermined origin, and to perform closed needle biopsies of the pleura only in young patients (in whom tuberculous pleurisy is more likely, at least in countries with relatively high prevalence of tuberculosis), and in those patients who reject thoracoscopy or are too sick to tolerate it, or in whom, due to adhesions, a pneumothorax of adequate size most probably cannot be induced (here closed needle biopsies can be attempted) (Antony et al. 2000; Rodriguez-Panadero et al. 2006). Tables 3.2 and 3.3 summarize the diagnostic and therapeutic advantages of MT/P.
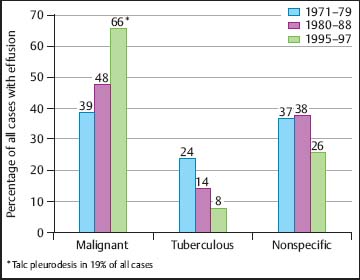
Fig. 3.5 Change in the etiology of pleural effusions that were examined thoracoscopically at the Lungenklinik Heckeshorn, Berlin during the last three decades of the twentieth century.
Figure 3.5 shows the changes in the etiology of pleural effusions in which MT/P was performed at Lungenklinik Heckeshorn during the previous three decades. Malignant effusions have now taken the lead and tuberculous effusions have decreased substantially, whereas the number of other pleural effusions has remained quite constant.
Malignant Pleural Effusions
Malignant pleural effusions are a common clinical problem in patients with neoplastic disease (Antony et al. 2000). They are one of the leading causes of exudative effusions; some studies have demonstrated that 42-77% of exudative effusions are secondary to malignancy (Marel et al. 1993; Valdés et al. 1996), but this may be due to patient selection. In one postmortem series, malignant effusions were found in 15% of patients who died with malignancies (Rodriguez-Panadero et al. 1989a).
Nearly all neoplasms have been reported to involve the pleura. In most studies, however, lung cancer has been the most common neoplasm, accounting for approximately one-third of all malignant effusions. Breast carcinoma is the second most common. Lymphomas, including both Hodgkin disease and non-Hodgkin lymphoma, are also an important cause of malignant pleural effusions. Tumors less commonly associated with malignant pleural effusions include ovarian and gastrointestinal carcinomas. In 5-10% of malignant effusions, no primary tumor is identified (Chernow and Sahn 1977; Johnston 1985; Antony et al. 2000). The incidence of mesothelioma varies according to the geographical location (Greillier and Astoul 2008).
Autopsy studies suggest that most pleural metastases arise from tumor emboli, seeding to the visceral pleural surface, with secondary seeding to the parietal pleura (Rodriguez-Panadero et al. 1989 a). Other possible mechanisms include direct tumor invasion (in lung cancers, chest-wall neoplasms, and breast carcinoma), hematogenous spread to the parietal pleura, or lymphatic involvement (Antony et al. 2000). A malignant tumor can cause a pleural effusion both directly and indirectly. Interference with the integrity of the lymphatic system anywhere between the parietal pleural and mediastinal lymph nodes can result in pleural fluid formation. Direct tumor involvement with the pleura may also contribute to the formation of pleural effusions. Local inflammatory changes in response to tumor invasion may cause increased capillary permeability, with resulting effusions.
The term “paramalignant effusions” is reserved for those effusions that are not the direct result of neoplastic involvement of the pleura but are still related to the primary tumor (Antony et al. 2000). Important examples include: postobstructive pneumonia with a subsequent parapneumonic effusion; obstruction of the thoracic duct, with the development of a chylothorax; pulmonary embolism; and transudative effusions secondary to post-obstruction atelectasis and/or low plasma oncotic pressures secondary to cachexia. Treatment of the primary tumor can also result in pleural effusions. Important causes in this category include radiation therapy and such drugs as methotrexate, procarbazine, cyclophosphamide, and bleomycin. Finally, concurrent nonmalignant diseases such as congestive heart failure may account for an effusion seen in a patient with cancer. MT/P offers the best chances for differentiation between malignant and paramalignant effusions in lung cancer patients compared with pleural fluid cytology and pleural carcinoembryogenic antigen (CEA) (Fig. 3.6).
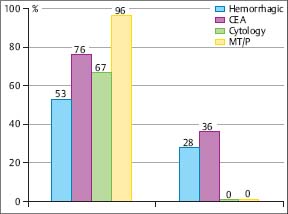
Fig. 3.6 Sensitivity (%) of different diagnostic criteria (hemorrhagic, CEA, cytology, MT/P) for differentiation between malignant (n = 67) and paramalignant (n = 25) pleural effusions in lung cancer patients at the Lungenklinik Heckeshorn, Berlin.
Most patients presenting with malignant pleural effusions have some degree of dyspnea on exertion, and their chest radiographs show moderate-to-large pleural effusions, ranging from approximately 500-2000 mL in volume (Antony et al. 2000). While only 10% of patients have massive pleural effusions on presentation, malignancy is the most common cause of massive pleural effusion, which is defined as occupying the entire hemithorax. About 15% of patients, however, will have pleural effusions < 500 mL in volume and will be relatively asymptomatic. The absence of contralateral mediastinal shift in these large effusions implies fixation of the mediastinum, main-stem bronchus occlusion by tumor (usually squamous-cell lung cancer), or extensive pleural involvement (as seen with malignant mesothelioma).
Computed tomography (CT) of patients with malignancies may identify previously unrecognized small effusions (Antony et al. 2000). It may also aid in the evaluation of patients with malignant effusions from mediastinal lymph node involvement and underlying parenchymal disease, as well as in demonstrating pleural, pulmonary, or distant metastasis. Identification of pleural plaques suggests asbestos exposure. Ultrasonography may aid in identifying pleural lesions in patients with malignant effusions and can be helpful in directing thoracentesis in patients with small effusions and avoiding thoracentesis complications.
Malignancy should be considered and a diagnostic thoracentesis performed in any individual with unilateral effusion or bilateral effusion and a normal heart size on the chest radiograph (Antony et al. 2000). It is reasonable to order the following pleural fluid tests when considering malignancy: cytology, nucleated cell count and differential, total protein, lactate dehydrogenase, glucose, pH, amylase, and tumor markers (optional).
Pleural fluid cytology is the simplest definitive method for obtaining a diagnosis of malignant pleural effusion (Antony et al. 2000). The diagnostic yield depends on such factors as extent of disease and the nature of primary malignancy. Studies have shown a large variation in diagnostic yields ranging from 22% to 94% (Heffner and Klein 2008). In some cases, differentiating between reactive mesothelial cells, mesothelioma, and adenocarcinoma can be problematic. Tumor markers such as carcinoembryonic antigen (CEA), Leu-1, and mucin may be helpful in establishing the diagnosis, as they are frequently positive in adenocarcinoma (50-90%) but rarely seen with mesothelial cells or mesothelioma (0-10%). Flow cytometry can complement cytology in some cases, particularly in lymphocytic effusions where lymphoma is suspected (Das 2006).
If the etiology remains unclear, invasive techniques are necessary to confirm the diagnosis. Closed needle biopsy of the chest wall pleura may establish the diagnosis in some additional cases, but usually adds little to the cytological diagnosis in most cases (Antony et al. 2000). This is related to the scars and irregular distribution of the tumor lesions in the pleural cavity when cytology is negative (Loddenkemper and Boutin 1993; Bielsa et al. 2008). In the series of 1000 consecutive patients with pleural effusions reported by Boutin and co-workers, 215 cases remained undiagnosed after repeated pleural fluid analysis and performance of pleural biopsies (Boutin et al. 1981a). This is in agreement with the results of several other authors, who, with or without the use of thoracoscopy, report that at least 20-25% of effusions remain undiagnosed (Storey et al. 1976; Hirsch et al. 1979; Lamy et al. 1980), although this certainly depends also on the selection of patient populations.
Most of the current guidelines recommend addition of a biopsy procedure when a first cytology is negative in effusions of unknown origin (Antony et al. 2000). Closed needle pleural biopsy of the costal (parietal) pleura is frequently advised in those cases. Closed needle biopsies have reported diagnostic yield of 40-75% in malignant pleural effusions. However, studies have shown that only 7-12% of patients with malignant pleural effusion may be diagnosed by pleural biopsy when fluid cytology is negative (Antony et al. 2000).
The relatively low yield of blind needle biopsy of the pleura is due to several factors, including early stage of disease with minimal pleural involvement, distribution of tumor in areas not sampled during blind biopsy, and operator inexperience. However, with the recent advances of imaging techniques some authors prefer CT-guided (Beau-champ et al. 1992; Metintas et al. 2010) or ultrasound (US)-guided (Chang et al. 1991) needle biopsies, which could replace blind needle biopsy in more than two-thirds of cases (Maskell et al. 2003), if abnormalities of the pleura can be identified with CT or US, as in mesothelioma. Cantó et al. studied the topography of pleural malignancies by medical thoracoscopy, and found that in 94% of their 203 cases the lower half of the pleural cavity was affected (Fig. 3.7). In 28% of cases, only the visceral pleura was involved and hence closed needle biopsy could not succeed (Cantó et al. 1983). Similar findings were seen in lung cancer (Cantó et al. 1985).
MT/P has a much higher diagnostic sensitivity and specificity than closed needle biopsy in malignant pleural effusions. Therefore, in cases of undiagnosed exudative effusions with a high clinical suspicion for malignancy, some clinicians may proceed directly to MT/P if cytology is negative and if the facilities for MT/P are available (Antony et al. 2000; Fletcher and Clark 2006). A theoretical cost analysis in the UK showed that MT/P saves considerable costs in unexplained pleural effusions compared with image-guided pleural biopsy (Medford 2010).
In a prospective intrapatient comparison, the diagnostic yield of the nonsurgical biopsy methods in malignant pleural effusions was studied simultaneously in 208 patients (Loddenkemper et al. 1983 a). These included: 58 diffuse malignant mesotheliomas; 29 cancers of the lung; 116 metastatic pleural effusions with 38 breast cancers; 30 cancers of various other origins; 58 of undetermined origin; and five malignant lymphomas. The overall diagnostic yield with cytological results from effusion was 62%, with needle biopsy (Tru-Cut) 44%, and with MT/P 95%, the latter showing a significantly higher sensitivity (p< 0.001) than needle biopsy with cytological results from effusions combined, which were positive in 74% of cases. All methods taken together were diagnostic in 97% of cases for malignant pleural effusion (Fig. 3.8). In six cases an underlying neoplasm was suspected at thoracoscopy but confirmed only by thoracotomy or autopsy in three cases each. Similar results were reported by several other investigators. In a study on 287 cases with malignant pleural effusion, there was virtually no difference in the yield of medical thoracoscopy for the different types of malignant effusions (Antony et al. 2000). The overall yield was 62% for cytology and 95% for MT/P; the yield for cytology and in particular thoracoscopy did not vary much between lung carcinomas (67% versus 96%, n = 67), extrathoracic primaries (62 % versus 95.5 %, n = 154), or diffuse malignant mesotheliomas (58% versus 92%, n = 66) (Fig. 3.9).
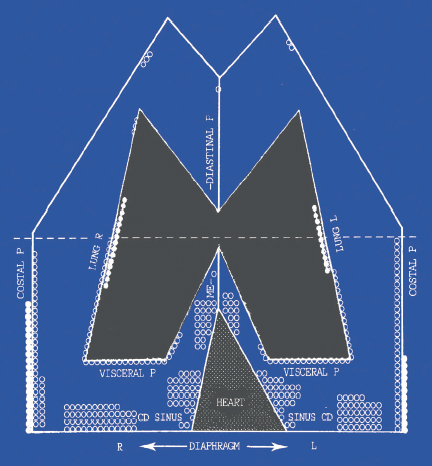
Fig. 3.7 Location of pleural metastases at thoracoscopy. (Reproduced with permission from Canto et al., Chest 1983;84:176-9.)
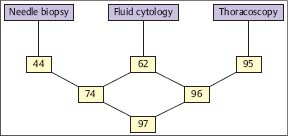
Fig. 3.8 Sensitivity (%) of different biopsy methods in malignant pleural effusions (prospective simultaneous comparison, n = 206) (Loddenkemper et al. 1983 b.)
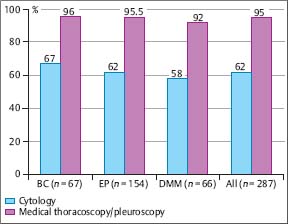
Fig. 3.9 Diagnostic yield (%) of cytology and medical thoracoscopy/pleuroscopy in malignant pleural effusions of different origin at the Lungenklinik Heckeshorn, Berlin 1980-1986. BC, bronchial carcinoma; EP, extrathoracic primary; DMM, diffuse malignant mesothelioma. (From Antony et al. 2001, reprinted with permission from ERS Journals Ltd.)
A further advantage of MT/P in malignant pleural disease is that biopsies can be taken under direct visual control not only of the costal pleura but also of the visceral and diaphragmatic pleura (Loddenkemper 1998). Since the thoracoscopic biopsies provide larger tissue specimens, this provides an easier histological/immunohistological identification of the primary tumor, including determination of hormone receptors in breast cancer (Levine et al. 1986; Schwarz et al. 2004), and a better morphological classification in lymphomas (Viallat et al. 1986; Celikoglu et al. 1992; Kawahara et al. 2008; Steiropoulos et al. 2009) and in their differential diagnosis (Schwarz et al. 2009). An additional advantage is that the diagnostic procedure can easily be combined with the therapeutic procedure of talc poudrage for pleurodesis (see “Thoracoscopic Talc Pleurodesis,” pp. 38–41, and Chapter 11, p. 93–94).
In addition, MT/P is helpful in staging of lung cancer, diffuse malignant mesothelioma, and metastatic cancers (Colt 1995 b). In lung cancer patients MT/P can determine whether the tumor has spread to the pleura, is secondary to venous or lymphatic obstruction, or is parapneumonic (Antony et al. 2000; Yoneda et al. 2007). As a result, it may be possible to avoid exploratory thoracotomy or to determine operability. Weissberg et al. (1981) performed thoracoscopy in 45 patients with lung cancer and pleural effusion. In 37 patients they found pleural invasion; three had mediastinal disease; the remaining five had no evident metastatic disease and, therefore, no contraindication to resection. Cantó and co-workers found similar results (Cantó et al. 1985). Of 44 patients, eight (18%) had no thoracoscopic evidence of pleural involvement, and six went to resection, where no tumor was found. A further study by Cantó et al. demonstrated that diagnostic sensitivity of malignancy was associated with the size of the effusion (Cantó et al. 1996). In conclusion, MT/P is the procedure of choice to differentiate between resectable and unresectable lung cancer if there is a pleural effusion present. In case of pleural metastasis, the stage of disease migrated previously to IIIB with a prognosis of stage IV (in the new staging system, malignant pleural effusion is classified as M1a and stage IV [Goldstraw et al. 2007]). In addition, the development of dedicated chemotherapy, even for metastatic non-small-cell lung cancer, warrants a specific diagnosis (Rodriguez-Panadero et al. 2006).
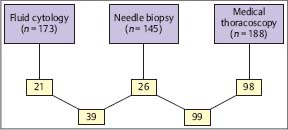
Fig. 3.10 Sensitivity (%) of different biopsy methods in diffuse malignant pleural mesothelioma. (Modified from Boutin and Rey 1993).
In diffuse malignant mesothelioma, the diagnostic yield of cytology is approximately 58%. MT/P can more often (Fig. 3.10) and more precisely provide a histological classification than closed pleural biopsy because of larger and more representative biopsies, along with more accurate staging (Boutin and Rey 1993; Boutin et al. 1993 b; Rusch 1995; Greillier and Astoul 2008; Scherpereel et al. 2010). This may have important therapeutic implications either for surgery or for local immunotherapy or local chemotherapy, since better results have been observed in the early stages(IandII)(Boutin et al. 1991; Astoul et al. 1993; Boutin et al. 1994; Nowaketal.2002; Vogelzang et al. 2003; Stewart et al. 2004; Sugarbaker et al. 2004). However, recent studies have shown that thoracoscopy may be less efficient in diagnosing the histological subtype (Bueno et al. 2004; Greillier et al. 2007). In histologically proven mesothelioma, the patient has a right to claim financial compensation from the former employer in several countries (Rodriguez-Panadero et al. 2006). In addition, fibrohyaline or calcified, thick, pearly-white pleural plaques may be found. MT/P helps in diagnosing benign asbestos pleural effusion (BAPE) by excluding mesothelioma or other malignancies with high accuracy (Boutin et al. 1998; Greillier and Astoul 2008). Thoracoscopic lung biopsies as well as biopsies from lesions on the parietal pleura may demonstrate high concentrations of asbestos fibers, providing further support for diagnosis of asbestos-induced disease (Boutin et al. 1996).
Reasons for false-negative MT/P include insufficient and nonrepresentative biopsies, which depend largely on the experience of the thoracoscopist, and the presence of adhesions that deny access to neoplastic tissue (Loddenkemper and Boutin 1993).
Autofluorescence videothoracoscopy with rigid instruments, developed by Richard Wolf GmbH in Germany, may help in the future to avoid some of the false-negative results (Chrysanthidis and Janssen 2005) (see Fig. 16.14 a, b in the Atlas section).
Narrow-band imaging during semirigid pleuroscopy, developed by Olympus Corporation, Japan, may also detect earlier discrete pathologic changes (Schönfeld et al. 2009) (see Fig. 14.9a, b in the Atlas section).
Another study, although under double-lumen intubation in general anesthesia, used fluorescence with 5-ami-nolevulinic acid in the diagnostic work-up for various pleural malignancies (D-LIGHT Auto Fluorescent System; Karl Storz, Germany). This method improved visualization of abnormal lesions and led to upstaging in four of 15 mesothelioma patients (Baas et al. 2006).
In a review of 4301 cases of diagnostic MT/P using rigid instruments in cases of chronic pleural effusion from 21 different studies, Boutin and co-workers reported that out of 1472 cancer cases a correct pathological diagnosis was achieved from thoracoscopic specimens in 1333 (92.5%). Conversely, 7.5% of thoracoscopic biopsies were false-negative (Boutin et al. 1991). Several explanations have been proposed:
1. In some cases of patients with cancer, pleural effusion is due not to malignant pleural effusion but to malignant obstruction of mediastinal or pulmonary lymphatics, which are not biopsied. This has been described both in lung cancer and cancer of the breast.
2. Rarely, an effusion is the late consequence of lymphatic obstruction due to mediastinal radiotherapy, which occurs in approximately 1% of cases (paramalignant pleural effusion).
3. The thoracoscopist learns with experience and has a suboptimal yield initially before full competence is achieved (a learning curve as experienced with all technical procedures).
4. Multiple biopsies must be taken systematically. There is no such thing as “too many.” The costovertebral gutter and the diaphragm must be routinely sampled. Metastases sometimes cannot be recognized endoscopically.
5. If the biopsy is reported as negative in suspected malignancy, the pathologist should be asked to section all tissue, including “deepest,” and to completely review all slides. This makes it possible to obtain additional diagnoses in some cases.
6. Sometimes the pleurae are covered with a fibrinous, necrotic layer, which requires removal to biopsy the parietal pleura behind it.
7. The major stumbling blocks for MT/P in cancer patients are cases of adherent pleura. The ability to obtain a biopsy depends on the practitioner’s skill in dividing and cutting adhesions, and there are some cases in which biopsy is impossible.
In their routine experience, they achieved 95-97% sensitivity in cancerous effusions, and false-negative findings were almost always due to adhesions that denied access to the neoplastic tissue.
The main additional advantage in using MT/P to diagnose malignancy is that talc poudrage can be performed in the same procedure, which is probably the most often used conservative option today for pleurodesis, with high success rates of more than 80% (Rodriguez-Panadero and Antony 1997; Tan et al. 2006a). An even distribution of the talc powder to all parts of the pleura can be achieved by poudrage under visual control. The extent of the carcinomatous involvement of the pleural cavity can be determined macroscopically, which allows some prediction of the success of pleurodesis: the intrapleural tumor spread can be semiquantitated using a scoring system (Table 3.4) that has been shown to correlate quite closely with survival and with the success of talc pleurodesis (Sanchez-Armengol and Rodriguez-Panadero 1993; Antony et al. 2004). Furthermore, by visual inspection it can be judged whether the lung will reexpand, which is an important prerequisite for a successful pleurodesis.
Tuberculous Pleural Effusions
In his pioneering publication in 1910Jacobaeus reported that he performed his first thoracoscopies in two patients with tuberculous pleural effusion (pleuritis exudativa) (Jacobaeus 1910). At that time, tuberculous pleurisy was one of the main causes of pleural effusion. Since then this has changed considerably in industrialized countries with low TB incidence. However, in some countries with a high TB prevalence, tuberculous pleurisy is still the leading cause of pleural effusions (Light 2010).
In Germany, for example, tuberculous pleural effusion now occurs in less than 5% of patients with TB (Forssbohm et al. 2008), but is more frequent (15-90%) in AIDS patients, with an effusion being more common in patients with higher CD4+ counts (Gopi et al. 2007). The diagnosis of pleural tuberculosis is hampered by its paucibacillary nature (Chegou et al. 2008). Less than 10% of pleural fluids are smear-positive and culture is positive in only approximately 30% (20-50%) of cases. The diagnostic yield of closed needle pleural biopsies is much better than in malignant pleural effusions due to the usually more disseminated involvement of the whole pleural surface. In a review of the literature on 1225 cases, the yield averaged 69% with a range of 28-88%, including results for multiple biopsies and TB cultures (Loddenkemper et al. 1983b). A study from South Africa showed that ultrasound-assisted needle biopsies were diagnostic for TB in 81.8% with the Abrams needles and in 65.2% with the Tru-Cut needles (Koegelenberg et al. 2009).
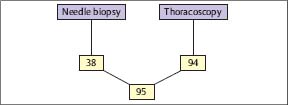
Fig. 3.11 Histological yield (%) of blind needle biopsy and medical thoracoscopy/pleuroscopy in tuberculous pleural effusions (prospective simultaneous comparison, n = 100). (Modified from Loddenkemper et al. 1983 b.)
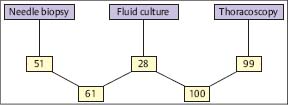
Fig. 3.12 Combined histological and bacteriological yield (%) of different biopsy methods in tuberculous pleural effusions. (Modified from Loddenkemper et al. 1983 b.)
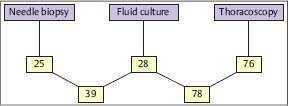
Fig. 3.13 Cultural yield (%) of different biopsy methods in tuberculous pleural effusions. (Modified from Loddenkemper et al. 1983 b.)
However, the diagnostic accuracy of MT/P is even better, because the pathologist is provided with multiple biopsies selected under visual control, and because the cultural proof of growth of TB bacilli is more often positive (Loddenkemper et al. 1978). MT/P therefore still has its value in the diagnosis of tuberculous pleurisy since it offers a high diagnostic sensitivity and specificity, and, in addition, a higher chance to determine drug resistance (Zarić et al. 2008).
In a prospective intrapatient comparison, an immediate diagnosis in 100 TB cases could be established histologically by MT/P in 94%, compared with needle biopsy (Tru-Cut) with only 38% positive results (Fig. 3.11) (Loddenkemper et al. 1983b). In individual cases, this may be of clinical importance because antituberculous chemotherapy can be started without delay. The combined yield of histology and bacteriological culture was positive for MT/P in 99% and for needle biopsy in 51 %, and when culture results from effusions were added, in 61 % of cases (Fig. 3.12). The percentage of positive TB cultures was twice as high from thoracoscopic biopsies, including cultures from fibrinous membranes (78%), which are always worth examining, as the percentage of cultures from pleural effusions and needle biopsies combined (39%) (Fig. 3.13).
Thus, MT/P much more often provides a bacteriological confirmation of the diagnosis of TB and, furthermore, the possibility of performing susceptibility tests. In five of the 78 positive cases (6.4%), resistance to one or more antituberculous drugs was found that had an impact on treatment and prognosis. It is of interest that the chance for a positive TB culture was much higher (87%) in cases were fibrin production in the pleural cavity was present. The typical changes with diffusely thickened pleura, multiple adhesions, and sometimes formation of encapsulating membranes with fluid loculations, were present in 76% of cases. By comparison, the pathognomonic picture of so-called “sagolike” pleuritis with miliary tuberculous granulomas and without fibrin layers was seen in only 24% of cases. In these cases, a positive TB culture was obtained from all biopsied specimens in only 50%, giving a highly significant difference (p < 0.0005).
Interestingly, the study also showed that the chance of positive TB cultures from pleural effusion alone was statistically much better in cases with a low pleural glucose level (< 50 mg/dL), indicating an increased metabolism of TB bacilli and/or a higher degree of inflammation (59% positive versus 25% with glucose levels above 50 mg/dL, p < 0.005). But the first group comprised only 17% of the patients, suggesting that they were diagnosed at quite an early stage. Cell count differentiation showed mainly lymphocytes in 71%, neutrophils in 9%, predominating in the early stages of growth without fibrin production, and in 20% in no characteristic cells at all.
In another prospective study on 40 cases from South Africa, MT/P had a diagnostic yield of 98% in comparison with an 80% diagnostic yield of Abrams needle biopsies (Walzl et al. 1996). This led to the conclusion that in areas with high prevalence of TB, closed needle biopsy alone—in this study three biopsies were obtained and each was examined histologically and microbiologically—can contribute significantly to the diagnosis of tuberculous pleurisy.
In a further study from the same institution in Stellen-bosch, South Africa, 51 patients with undiagnosed exudative pleural effusion were recruited for a prospective, direct comparison between bronchial wash, pleural fluid microbiology, biochemistry (adenosine deaminase [ADA]), cell count, closed needle biopsy, and MT/P (Diacon et al. 2003). The final diagnosis was TB in 41 patients (82%). The sensitivities of histology, culture, and combined histology/culture were 66%, 48%, and 79%, respectively, for closed needle biopsy, and 100%, 76%, and 100%, respectively, for MT/P. Since the combination of ADA, lymphocyte/neutrophil ratio ≥ 0.75 plus closed needle biopsy reached 93% sensitivity and 100% specificity, the authors concluded that this high diagnostic accuracy is sufficient in an area with a high incidence of tuberculosis. However, if this combination of tests is negative despite a high clinical suspicion of tuberculous pleurisy, if antibiotic resistance is of concern, or if other possible diagnoses are considered, they recommend MT/P as the method of choice for the final diagnosis.
A retrospective study from Japan achieved similar results in 32 patients with tuberculous pleurisy. Semirigid pleuroscopic biopsies were positive in 93.8% with histological and bacteriological examination and in 65.6% with pathological examination alone (Sakuraba et al. 2006 a).
Several laboratory and microbiological tests have been developed during recent years, including nucleic acid amplification and detection, markers of specific and nonspecific immune response such as ADA, and T cell-based inter-feron-gamma release assays (IGRAs), which may allow the diagnosis of TB as the cause of pleural effusion with high sensitivity and specificity (Trajmanetal.2008; Light 2010). However, at least in countries with low prevalence of TB, the accuracy may not be sufficient in individual cases (Hooper et al. 2009). This is underlined by a further study from Japan in which 138 patients underwent MT/P for unexplained pleural effusion (Sakuraba et al. 2009). Of 50 patients who had effusions with ADA levels of less than 50 IU/L, a tuberculous pleurisy was diagnosed in six patients (12%) by thoracoscopic biopsy. These patients would have been diagnosed with nonspecific pleurisy otherwise. It is questionable whether patients should undergo treatment with antituberculous drugs merely on the suspicion of a tuberculous etiology of the pleurisy. In our opinion, in these cases MT/P should be performed to prove or exclude TB.
A further important argument in favor of MT/P is provided by the fact that thoracoscopic biopsies much more often yield positive TB cultures (see above, Loddenkemper et al. 1983b), which give rise to the possibility of obtaining susceptibility tests that, in particular in patients born in countries with high resistance rates, especially of multi-drug resistance (MDR) or extensive drug resistance (XDR) (World Health Organization 2009), may have a considerable impact on the correct treatment and the final outcome after therapy (Baumann et al. 2007; Gopi et al. 2007).
In addition, the immediate and complete drainage of the pleural fluid, achieved during and after MT/P, is associated with greater and direct symptomatic improvement than is any subsequent therapy. This was demonstrated in a further study from Stellenbosch on the effect of corticosteroids on the treatment of TB pleurisy (Wyser et al. 1996). Up to now, no studies have been done that compare the impact of MT/P with its fast diagnosis, complete drainage, and subsequent early drug treatment to a group of patients with drug treatment alone.
Although some authors are not much concerned with long-term complications of tuberculous effusions (Sahn 2002), the positive role of early removal of the pleural fluid has been shown recently in a double-blind, randomized, placebo-controlled trial from Taiwan (Chung et al. 2008). In this study, it was demonstrated that early effective drainage, combined with complete antituberculous treatment, may hasten clearance of pleural effusion, reduce residual pleural thickening occurrence, and accelerate pulmonary function recovery in patients with symptomatic loculated tuberculous pleural effusion who were irrigated in addition with streptokinase instead of saline alone. This is in accordance with the experience at Lungenklinik Heckeshorn where, after early MT/P with complete evacuation of the pleural fluid—if necessary combined with mechanical opening of loculations—and subsequent pleural drainage, no single case of tuberculous pleurisy was observed in 20 years that needed surgical de-cortication for fibrothorax.
Parapneumonic Effusions and Pleural Empyema
The optimal management of parapneumonic effusions (PPE) and empyema in the fibrinopurulent state (stage II) is still controversial, with a lack of controlled studies, whereas the organizational stage (stage III) is reserved almost exclusively for surgical interventions, since this stage shows continued fibroblast migration and the production of pleural fibrosis with lung trapping (Colice et al. 2000). The initial evaluation should focus on three critical questions: (1) Should the pleural space be drained? (2) How should the pleural space be drained? (3) Should fibrinolytics be instilled? (Koegelenberg et al. 2008).
Treatment goals in stage II, besides eradication of the causative pathogen, include evacuation of all (infected) pleural fluid, prevention or breakdown of (multi-)loculations, and prevention of lung trapping by fibrin deposition on the visceral and parietal pleura. This is rarely achieved by antibiotic treatment alone, but needs pleural space drainage in almost all cases. Local pleural treatment options can be separated into medical (conservative) and surgical (Loddenkemper et al. 2004). The medical options include (serial) therapeutic thoracentesis, small-bore tubes, or standard chest tubes, often combined with instillation of fibrinolytic agents and/or irrigation of the pleural cavity. This can be combined with MT/P. Arguments in favor of conservative treatment are that (1) it is less invasive and (2) it can be applied even in severely sick patients (with sepsis, on ventilator, in the intensive care unit, on immunosuppressive disease/treatment, or in other severe concomitant diseases).
Table 3.5 Role of medical thoracoscopy/pleuroscopy in the management of complicated parapneumonic effusion/empyema
• Technique similar to chest tube insertion • Direct visualization of loculations • Inspection and characterization of the pleural surface • Breakdown of fibrinous septae and creation of one single pleural space Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|