We correlated von Willebrand factor (VWF) activity indexes and brain natriuretic peptide (BNP) with measures of aortic stenosis (AS) severity, bleeding, symptoms, and freedom from death or aortic valve replacement. Patients with AS (n = 66 [16 mild, 20 moderate, and 30 severe]) and aortic valve replacement (n = 21) were assessed with VWF antigen, VWF latex agglutination immunoturbidic activity, platelet function analyzer collagen plus adenosine diphosphate (PFA-CADP), VWF multimer ratio, and BNP level after echocardiography. In patients with AS, the mean gradient correlated with BNP (Spearman r = 0.29, p = 0.02), VWF latex agglutination immunoturbidic activity/VWF antigen ratio (r = −0.41, p <0.001), PFA-CADP (r = 0.49, p <0.001), and VWF multimer ratio (r = −0.76, p <0.001). The area under the curve for detection of severe AS was 0.62 (95% confidence interval [CI] 0.48 to 0.77) by elevated BNP, 0.81 (95% CI 0.69 to 0.92) by PFA-CADP closure time, 0.69 (95% CI 0.55 to 0.82) by VWF latex agglutination immunoturbidic activity/VWF antigen ratio, and 0.86 (95% CI 0.76 to 0.95) by VWF multimer ratio. For the VWF multimer ratio, a threshold of 0.15 yielded a sensitivity and specificity for severe AS of 77% and positive predictive value of 74%. Bleeding (in 14%) was associated with a prolonged PFA-CADP time and reduced VWF latex agglutination immunoturbidic activity/VWF antigen ratio. Symptoms were associated with elevated BNP and low Duke Activity Status Index score. In 66 patients with AS, freedom from death (n = 4) or aortic valve replacement (n = 22) was associated with PFA-CADP (p = 0.003), VWF high-molecular-weight multimers (p = 0.009), and VWF latex agglutination immunoturbidic activity/VWF antigen ratio (p <0.001) but not BNP (p = 0.32). In severe AS versus aortic valve replacement, the PFA-CADP and VWF multimer ratio differed (p <0.001), but BNP and the VWF latex agglutination immunoturbidic activity/VWF antigen ratio did not. In conclusion, the VWF activity indexes were associated with AS severity and bleeding and were predictive of cardiovascular outcomes.
Decision making for patients with aortic stenosis (AS) is confounded by overlapping moderate and severe designations and uncertainty whether symptoms might be undetected or originate from other clinical conditions. The various echocardiographic severity criteria have been inconsistent in the assessments of prognosis. The case for the use of a biomarker that reflects disease severity, reliably detects progression, and assesses the prognosis and response to therapy was made in a recent comprehensive review of natriuretic peptides by Steadman et al. There is a strong graded relation between the functional abnormalities of von Willebrand factor (VWF) and AS gradient in surgical cases and in patients with hypertrophic cardiomyopathy with obstruction and the correction of laboratory VWF functional abnormalities and clinical bleeding tendency with aortic valve replacement or septal myectomy for hypertrophic cardiomyopathy. Therefore, we sought to assess the performance of VWF activity indexes as biomarkers of AS severity across a broad range of disease in patients who had undergone aortic valve replacement and compared these findings with those for brain natriuretic peptide (BNP).
Methods
Patients referred for echocardiography with AS, peak transvalvular velocity >2.5 m/s, or aortic valve replacement were eligible. Clinical data were collected, and a questionnaire on bleeding modified from the consensus conference on the assessment of von Willebrand disease ( Supplemental Table 1 ) and the Duke Activity Status Index ( Supplemental Table 2 ) were administered prospectively. The exclusion criteria were inadequate images, hemoglobin <8 g/dl, thienopyridine therapy, or severe left heart regurgitant lesions or mitral stenosis. The outcomes were determined by medical record review and/or interview by telephone to establish the vital status and date of surgery or death. All patients provided written informed consent, and the study complied with the Declaration of Helsinki and was approved by the Mayo Clinic institutional review board (Clinical Trial Registration: NCT no. 01334801 ).
The echocardiographic assessment of AS followed the American Society of Echocardiography guidelines. Wall shear was calculated as 4 times the blood viscosity estimated at 0.035 poise times the mean transvalvular blood velocity divided by the radius of the stenosis. Correlations between the biomarkers and all usual measures of AS severity and wall shear stress were calculated; however, for associations with bleeding risk, symptoms, and high-molecular-weight multimer (HMWM) loss, we used the mean aortic gradient >40 mm Hg as the definition of severe AS, 25 to 40 mm Hg as moderate AS, and <25 mm Hg as mild AS.
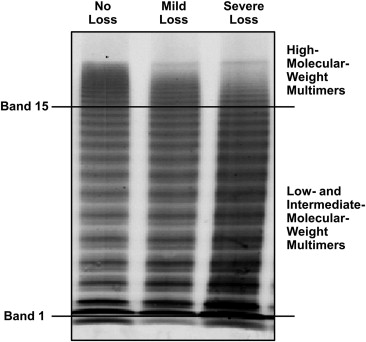
Citrated plasma was stored frozen until testing, usually after 1 to 3 months. VWF antigen and VWF latex agglutination immunoturbidic activity are both expressed as a percentage of the control, as previously described. The primary clinical interpretation of VWF multimers at our institution is determined by a visual inspection of the patients’ plasma and control pooled plasma ( Figure 1 ) recorded as the subjective loss of HMWMs or normal HMWM levels. This was recorded as a dichotomous variable. Quantification of VWF multimers, a research tool at our institution, involved scanning of raw fluorescent intensity of patients’ multimer electrophoresis using an Odyssey Infrared Imager, version 2.0 (LI-COR, Lincoln, Nebraska), enclosing bands 2 to 15 containing low-molecular-weight multimers and another adjacent one enclosing intermediate-molecular-weight multimers and HMWMs, bands 16 to 20 ( Figure 1 ). The fluorescent intensity of bands 16 to 20 divided by that of bands 2 to 15 was designated as the VWF multimer ratio and recorded as a continuous variable. We did not include the first band in our calculations because of significant artifact associated with this band, such as can be seen in samples from type 3 von Willebrand disease. The platelet function analyzer collagen plus adenosine diphosphate (PFA-CADP) closure time (Siemens USA, Washington, DC) was determined. The reference interval in the laboratory was 57 to 121 seconds.
The BNP immunoenzymatic assay (BioSite, San Diego, California) was performed using the patients’ fresh serum samples (Beckman Coulter DXI 800 immunoassay system, Beckman Coulter, Brea, California). The normal values for this assay were age- and gender-adjusted (women have considerably greater normal values than men). In general, values of 200 to 400 ng/L suggested moderate heart failure, and those >400 ng/L indicated moderate to severe heart failure.
Patients were defined as having nonclinically significant bleeding if they had all negative questionnaire responses or if they had incidental bruising, epistaxis, or blood in stool but no clinically relevant events in their medical history for the past 3 years; had received remote surgery-related transfusion; or were postmenopausal women who reported remote menorrhagia. The Duke Activity Status Index is a 12-point activity index that yields a raw score and an estimate of maximum exercise oxygen consumption.
The associations of biomarkers with echocardiographic measures of AS severity were estimated using the Spearman rank correlation coefficient (r) and bootstrapped 95% confidence intervals (CIs). Receiver operating characteristic curves were constructed to explore the diagnostic utility of these biomarkers for severe AS (defined as a mean gradient >40 mm Hg). The area under the curves and 95% CIs were estimated. A comparison of the areas under the curve of each VWF biomarker to that of BNP was performed on the basis of methods proposed by DeLong et al. The sensitivity and specificity with exact binomial 95% CIs were estimated for BNP greater than each individual upper limit of normal value, PFA-CADP closure time >121 seconds, VWF latex agglutination immunoturbidic activity/VWF antigen ratio <0.8, and VWF multimer ratio >0.15.
In a secondary analysis, Kaplan-Meier curves estimating freedom from death and aortic valve replacement were constructed on the basis of predetermined cutoff values; age-adjusted hazard ratios (HRs) from Cox regression models were estimated. Comparisons between patient groups were based on the Mann-Whitney U test for numerical variables and the Fisher exact test for categorical variables. Because of the exploratory nature of the analyses, adjustment for multiple testing was not done. p Values <0.05 were considered statistically significant. Analyses were performed using SAS, version 9.2 (SAS Institute, Cary, North Carolina) and R Statistical Software, version 2.14.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 87 patients had research biomarkers obtained (32 women, 57 men, 66 with AS and 21 with aortic valve replacement [11 mechanical and 10 bioprosthetic]). In 65 patients (75%), biomarkers were obtained within 48 hours of the echocardiogram. The plasma of 1 patient with AS was processed incorrectly for VWF measures; however, the PFA-CADP and BNP tests were performed correctly. The baseline characteristics of the 66 patients with AS are summarized in Table 1 . Thirty-one patients (47%) had physician-identified AS-related symptoms. During a median duration of 11.7 months, 4 patients with AS died and 22 underwent aortic valve replacement.
| Variable | AS (n = 66) |
|---|---|
| Men | 43 (65%) |
| Age (yrs) | 79 (70–83) |
| Hypertension | 46 (70%) |
| Glomerular filtration rate (ml/min) | 60 (58–60) |
| Documented coronary disease | 15 (23%) |
| Stroke or emboli | 8 (12%) |
| Diabetes mellitus | 14 (21%) |
| Atrial fibrillation | 14 (21%) |
| Heart failure | 11 (17%) |
| Current or previous smoker | 43 (65%) |
| Aspirin use | 41 (62%) |
| Statin use | 32 (48%) |
| Symptoms of aortic stenosis | 31 (47%) |
| Left ventricular hypertrophy (electrocardiography) | 22 (33%) |
Included as a control group were 21 patients who had previously undergone aortic valve replacement. Their median age was 81 years (interquartile range 76 to 86). Compared to the 30 patients with severe AS, those who had undergone aortic valve replacement had a significantly greater VWF multimer ratio (median 0.173 vs 0.128; p <0.001), had a shorter PFA-CADP closure time (median 92 vs 187 seconds; p <0.001), and were less likely to have a loss of HMWMs (10% vs 87%; p <0.001). The BNP level and VWF latex agglutination immunoturbidic activity/VWF antigen ratio were not significantly different between those with severe AS and those with aortic valve replacement ( Table 2 ). The indexed valve areas in the valve replacement patients, not corrected for pressure recovery, suggested the presence of patient prosthesis mismatch (aortic valve area index ≤0.65 cm 2 /m 2 ) in 5 of 21 patients, 1 of whom had an VWF multimer ratio <0.15, and none with loss of HMWMs.
| Variable | Severe AS (n = 30) | Aortic Valve Replacement (n = 21) | p Value |
|---|---|---|---|
| Mean gradient (mm Hg) | 51 (45–62) | 15 (12–19) | <0.001 |
| Peak aortic velocity (m/s) | 4.50 (4.30–4.93) | 2.46 (2.40–2.80) | <0.001 |
| Brain natriuretic peptide (ng/L) | 177 (74–592) | 203 (115–369) | 0.84 |
| Elevated brain natriuretic peptide | 21 (70%) | 17 (81%) | 0.52 |
| Platelet function analyzer adenosine closure time (s) | 187 (128–223) | 92 (82–106) | <0.001 |
| von Willebrand factor latex activity/von Willebrand factor antigen ratio | 0.87 (0.76–0.94) | 0.96 (0.86–1.00) | 0.09 |
| von Willebrand factor multimer ratio | 0.128 (0.110–0.149) | 0.173 (0.162–0.185) | <0.001 |
| Loss of von Willebrand factor high-molecular-weight multimers | 26 (87%) | 2 (10%) | <0.001 |
Loss of HMWMs was detected in 37 of the 65 patients with AS ( Table 3 ), which was highly associated with AS severity, as indicated by the mean gradient (p <0.001), peak aortic velocity (p <0.001), and wall shear stress (p = 0.001). Those with versus without HMWM loss had a lower VWF latex agglutination immunoturbidic activity/VWF antigen (median 0.887 vs 0.956; p = 0.02) and VWF multimer (median 0.132 vs 0.171; p <0.001) ratios. Of the 47 patients with VWF multimers interpreted as normal, 10 had an VWF multimer ratio <0.15. Of the 39 patients with VWF multimers interpreted as abnormal, 10 had an VWF multimer ratio >0.15. The loss of HMWMs was associated with longer PFA-CADP closure times (median 151 vs 107 seconds; p = 0.004) and Cornell electrocardiographic left ventricular hypertrophy scores (median 2,720 vs 1,665; p = 0.001). HMWM loss was not associated with clinical symptoms, BNP level, or clinically significant bleeding.
| Variable | HMWM | p Value | |
|---|---|---|---|
| Normal (n = 28) | Loss (n = 37) | ||
| Mean gradient (mm Hg) | 26 (19–34) | 46 (38–56) | <0.001 |
| Peak aortic velocity (m/s) | 3.3 (2.8–3.9) | 4.4 (3.9–4.8) | <0.001 |
| Aortic valve area index (cm 2 /m 2 ) | 0.68 (0.43–0.82) | 0.43 (0.37–0.60) | 0.005 |
| Wall shear stress (dynes/cm 2 ) | 58 (39–72) | 90 (68–108) | <0.001 |
| Significant bleeding | 2 (7%) | 7 (19%) | 0.28 |
| Symptoms | 12 (43%) | 19 (51%) | 0.62 |
| Duke Activity Status Index score | 36 (18–51) | 35 (19–50) | 0.57 |
| Cornell left ventricular hypertrophy score | 1,665 (1,077–2,568) | 2,720 (1,950–3,268) | 0.001 |
| Brain natriuretic peptide (ng/L) | 150 (67–240) | 122 (74–386) | 0.73 |
| Platelet function analyzer collagen plus adenosine diphosphate closure time (s) | 107 (83–134) | 151 (104–214) | 0.004 |
| von Willebrand factor latex activity/von Willebrand factor antigen ratio | 0.956 (0.881–1.052) | 0.887 (0.796–0.934) | 0.02 |
| von Willebrand factor multimer ratio | 0.171 (0.155–0.186) | 0.132 (0.110–0.157) | <0.001 |
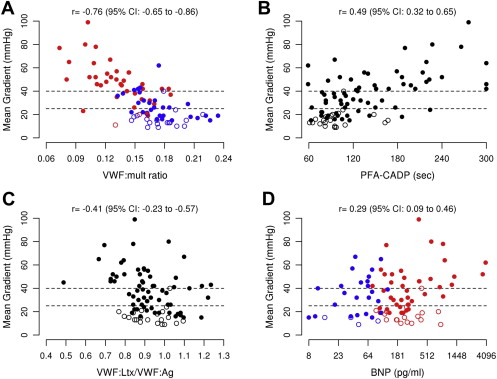
The Spearman rank correlations of the biomarkers with the mean AS gradient are listed in Table 4 . The VWF multimer ratio demonstrated the strongest relation to the mean gradient (r = −0.76, 95% CI −0.65 to −0.86, p <0.001; Figure 2 ). Significant associations with the mean gradient were demonstrated for BNP (p = 0.02), PFA-CADP (p <0.001), and VWF latex agglutination immunoturbidic activity/VWF antigen ratio (p <0.001; Figure 2 ). Similar results were found when examining the correlations between the plasma biomarkers and other echocardiographic measures of AS.
| Echocardiographic Measurements | Plasma Biomarkers | |||||||
|---|---|---|---|---|---|---|---|---|
| BNP (ng/L) | PFA-CADP (s) | VWF Latex Activity/VWF Antigen Ratio | VWF Multimer Ratio | |||||
| r | p Value | r | p Value | r | p Value | r | p Value | |
| Mean gradient (mm Hg) | 0.29 | 0.02 | 0.49 | <0.001 | −0.41 | <0.001 | −0.76 | <0.001 |
| Peak aortic velocity (m/s) | 0.25 | 0.04 | 0.50 | <0.001 | −0.41 | <0.001 | −0.73 | <0.001 |
| Aortic valve area (cm 2 ) | −0.51 | <0.001 | −0.51 | <0.001 | 0.39 | 0.001 | 0.57 | <0.001 |
| Aortic valve area index (cm 2 /m 2 ) | −0.47 | <0.001 | −0.51 | <0.001 | 0.41 | <0.001 | 0.57 | <0.001 |
| Aortic valve time velocity integral (cm) | 0.34 | 0.005 | 0.57 | <0.001 | −0.34 | 0.006 | −0.70 | <0.001 |
| Time velocity integral ratio | −0.45 | <0.001 | −0.51 | <0.001 | 0.34 | 0.006 | 0.56 | <0.001 |
| Wall shear stress (dynes/cm 2 ) | 0.40 | <0.001 | 0.55 | <0.001 | −0.40 | 0.001 | −0.70 | <0.001 |
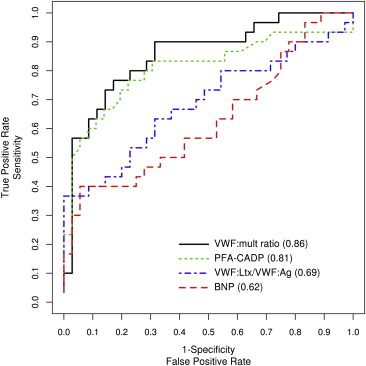
In the detection of severe AS according to a mean gradient >40 mm Hg, the estimates of the area under the curve ( Figure 3 ; Supplemental Table 2 ) showed that the PFA-CADP closure time and VWF multimer ratio were best at detecting severe AS, with an area under the curve of 0.81 and 0.86, respectively. The area under the curve for VWF latex agglutination immunoturbidic activity/VWF antigen ratio and BNP was 0.69 and 0.62, respectively. Compared to BNP, the VWF multimer ratio had a significantly greater area under the curve in the detection of AS (p = 0.004); however, neither PFA-CADP nor VWF latex agglutination immunoturbidic activity/VWF antigen had a significantly greater area under the curve than BNP in the detection of AS (p = 0.06 and p = 0.45, respectively). In an exploratory analysis of possible cutoff points of VWF multimer ratio ( Supplemental Table 3 ), a threshold of 0.15 demonstrated a 77% sensitivity, 77% specificity, and 74% positive predictive value for the detection of a mean gradient >40 mm Hg.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


