Many adult survivors of repaired congenital heart disease (CHD) are at premature risk of death. Sudden cardiac arrest (SCA) is 1 of the leading causes of death but little is known about determinants for SCA in adults with repaired lesions. We sought to determine incidence and risk factors for SCA in a study population of 936 adults with previously repaired CHD who had completed follow-up at a single tertiary center during a mean period of 9 ± 7 years. Mean age at first examination in our institution was 21 ± 7 years. Diagnostic categories included tetralogy of Fallot (216), coarctation of the aorta (157), transposition complexes (99), single ventricle (55), and other CHD (409). During a total follow-up of 8,387 person-years, 22 patients (2.6 per 1,000 person-years) presented with SCA. Incidence of SCA varied widely between specific lesions; the highest incidence was observed in transposition complexes (10 per 1,000 person-years). Independent predictors of SCA were retrospectively identified using multivariate Cox proportional hazard modeling. Age at initial examination and severely impaired subaortic ventricular systolic function were independent risk factors for SCA (severe subaortic ventricular systolic dysfunction, adjusted hazard ratio 29, 95% confidence interval 11 to 72, p <0.001). SCA occurred in 23% of patients with severe subaortic ventricular systolic dysfunction versus 0.7% of patients with nonsevere decreased subaortic ventricular function (p <0.001). In conclusion, severe subaortic ventricular systolic dysfunction is a dominant multivariate predictor of SCA in an unselected population of adult survivors after surgery for CHD. Our data support the consideration of primary prevention strategies in these patients.
Long-term mortality for patients with congenital heart disease (CHD) has been assessed in several population-based studies or multicenter registries. Sudden cardiac arrest (SCA) is 1 of the leading causes of death in patients with CHD, and approximately 73% of sudden deaths are estimated to be arrhythmic. Although the reported annual incidence of SCA in the total CHD population is low, it represents an increased risk of 25 to 100 times that of the general population. Knowledge of predictors of SCA in CHD would facilitate clinical decision making of which patients might benefit from prophylactic therapies. Most studies seeking risk factors for SCA have focused on adults with repaired tetralogy of Fallot (TOF) and adults after Mustard/Senning operation for transposition of the great arteries (TGAs). Because of these studies, many individual factors have been proposed as predictors of late SCA or malignant arrhythmias and subaortic ventricular function has gained increasing attention as a potential risk factor for SCA. However, it is unclear whether these results can be applied to postoperative patients with other congenital defects. We sought to determine the incidence rate and predictors of SCA in a large single-center cohort of adults with repaired CHD.
Methods
Clinical records of 3,197 patients referred to the adult CHD unit at La Paz University Hospital for cardiac evaluation from January 1990 through January 2010 were retrospectively evaluated. For the present study, 1,289 patients (40%) who had a reparative or palliative intervention for CHD at <20 years of age were identified. The study group included all patients who were followed serially at our institution with a clinical visit within the previous 2 years or before an end point. A comprehensive assessment of patients including cardiac imaging examination and cardiac catheterization as appropriate had been performed at the last clinical visit. Patients for whom a clinical visit at our adult CHD unit did not occur within the previous 2 years and whose clinical status was unknown were excluded from analyses. The study was approved by the local research ethics committee.
CHD lesions were divided into diagnostic categories: (1) repaired TOF including complex forms of this defect such as pulmonary atresia with nonrestrictive ventricular septal defect; (2) repaired coarctation of the aorta by surgery or percutaneous balloon angioplasty or stenting; (3) TGA after Senning, Mustard, Rastelli, and/or Jatene procedures; (4) surgical or percutaneous relief of valvular, discrete subvalvar, and supravalvar aortic stenosis; (5) repaired obstructive right ventricular outflow tract lesions after surgical or balloon valvulotomy or patch reconstruction; (6) surgically repaired atrioventricular septal defect (including primum atrial septal defect); (7) surgically repaired ventricular septal defect; (8) repaired ostium secundum or sinus venosus defect; (9) anatomic or functional single-ventricle physiology after Fontan palliation; (10) single-ventricle physiology after aortopulmonary shunts or Glenn shunt palliation; (11) patent ductus arteriosus repaired by surgery or catheterization; and (12) congenitally corrected TGA after surgical repair of a ventricular septal defect, left ventricular outflow tract obstructive lesions, or left atrioventricular valve dysfunction. Diagnoses that did not belong in 1 of these 12 diagnostic categories such as total pulmonary venous anomalous connection or coronary anomalies were categorized as miscellaneous. If >1 diagnostic category applied, the hemodynamically most important lesion for each patient regarding long-term outcome was classified as the principal diagnosis. If >1 lesion was considered to have a major impact on morbidity, the driver to assign the principal diagnosis was the lesion considered the indication for the initial surgical approach.
Details of demographic characteristics, clinical status, Doppler echocardiography, cardiac magnetic resonance imaging, and cardiac catheterization were abstracted from patients’ clinical records. The most recent data preceding the end points were requested. Evaluation of systolic ventricular function was based on M-mode and 2-dimensional echocardiographic measurements and expert visual assessment supplemented by objective measurements including ventricular diameters, fractional shortening, tricuspid or mitral annular plane systolic excursion, and tissue Doppler parameters. Because ventricular function in the morphologic right ventricle and for single-ventricle physiology may be assessed inaccurately by echocardiography, for the previous 10 years those patients with significantly decreased subaortic or subpulmonary right ventricular systolic function underwent cardiac magnetic resonance whenever possible to confirm the severity of ventricular dysfunction. Subaortic and subpulmonary systolic ventricular functions were visually graded by echocardiography in 4 degrees: (1) normal (ejection fraction ≥55%), (2) borderline to mild (ejection fraction 45% to 54%), (3) moderate (ejection fraction 35% to 44%), and (4) severe (ejection fraction <35%) dysfunction. Consistent with recently modified recommendations for implantable cardioverter–defibrillator (ICD) implantation in heart failure, an ejection fraction <35% was used in this study to define severe impairment.
For univariate and multivariate models, the following variables were assessed: (1) gender; (2) age at first examination in our adult CHD clinic; (3) age at last examination or at end-point event; (4) time from first visit to any end point or to last visit; (5) New York Heart Association functional classes I to II or >II at the last examination; (6) clinically sustained arrhythmias such as supraventricular or ventricular tachycardia, atrioventricular block, or sinus node disease that required pacemaker implantation (nonsustained arrhythmias captured from Holter monitoring or data on ambient ectopy were not considered in analysis); (7) infective endocarditis during adult follow-up; (8) need for surgical or percutaneous reintervention during follow-up; (9) severely impaired systolic function of the subaortic ventricle at last cardiac imaging study; (10) severely impaired subpulmonary ventricular function at last cardiac imaging study; (11) subaortic right ventricle (single or biventricular physiology); (12) severe pulmonary hypertension defined as a pulmonary-to-systemic systolic pressure ratio >50% on cardiac catheterization or Doppler evaluation; (13) severe pulmonary regurgitation assessed by echocardiography or cardiac magnetic resonance; (14) severe aortic regurgitation assessed by echocardiography or cardiac magnetic resonance; (15) severe subaortic atrioventricular valve regurgitation assessed qualitatively by Doppler echocardiography; and (16) severe subpulmonary atrioventricular valve regurgitation assessed qualitatively by Doppler echocardiography. Diagnostic categories were also entered into the models.
According to previous publications, cause of death was classified as sudden death, death secondary to heart failure, perioperative death, other cardiovascular death, or noncardiovascular death. Other causes of cardiovascular death included aortic rupture, endocarditis, acute myocardial infarction, pulmonary embolism, and stroke. The primary end point was SCA defined as the combined end point of sudden death or aborted cardiac arrest. Sudden death was defined as death within 1 hour of symptom onset and proved or believed to be arrhythmic in nature. Aborted cardiac arrest included successful cardiopulmonary resuscitation of a cardiovascular collapse resulting from ventricular fibrillation and documented appropriate ICD discharge delivered in response to polymorphic ventricular tachycardia or ventricular fibrillation. A secondary composite end point included all-cause of death and heart or heart–lung transplantation.
SPSS 15.0 for Windows (SPSS, Inc., Chicago, Illinois) was used for statistical analyses. Quantitative values are summarized as mean ± SD. Categorical variables are presented as percentage. For purposes of the analyses, age and time computed since the first visit at our clinic were considered continuous variables and all other variables were considered categorical. Patients fulfilling inclusion criteria were retrospectively included in the analysis at the time of their first visit at the adult CHD clinic during the study period. Person-years were accrued from time of entry until an end point or until the last follow-up visit before study termination. Prevalence was presented as percentage and defined as total number of events that occurred during total follow-up period divided by total number of cases or by number of patients in each diagnostic category. Incidence was measured by relating the number of new events to person-years at risk and calculated by summing the periods during which patients in the total population or in each diagnostic category were at risk during the observation period. It was represented by events per 1,000 person-years. Comparison of variables between groups was performed with Mann–Whitney U test. To assess predictors for SCA in longitudinal analysis, univariate analyses of individual variables were performed using Cox proportional hazards models from which hazard ratios and 95% confidence intervals were generated. To determine independent risk factors, variables with a p value <0.2 in univariate analysis were entered into the multivariate Cox proportional model (forward stepwise, p = 0.05 for entry and p = 0.1 for removal). Two-tailed p values <0.05 were considered statistically significant.
Results
In total 936 patients (73%) were eligible for inclusion and comprised the study population. Distribution of diagnostic categories and duration of follow-up for the entire sample and separately by CHD category are presented in Table 1 . Although the mean follow-up period ranged from 5 ± 5 years in patients with atrial septal defects to 11 ± 8 years in patients with repaired aortic stenosis, differences in follow-up were not statistically significant between diagnostic categories. Further details on patient characteristics are presented in Table 2 . It should be noted that 83% of patients with TGA repaired by a Mustard/Senning procedure plus 10 patients with congenitally corrected TGA and 7 of 55 patients with a single ventricle had morphologic right ventricles supporting their systemic circulation.
| Diagnostic Category | Cases (%) | Men | Age (years) | Follow-Up (years) | SCA | Death or Transplantation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial Examination (%) | Last Follow-Up | Mean ± SD | Person-Years | Cases (%) | Incidence/1,000 Person-Years | Age at Sudden Arrest | Cases (%) | Incidence/1,000 Person-Years | Age at End Point | |||
| Tetralogy of Fallot | 216 (23%) | 112 (52%) | 22 ± 9 | 32 ± 8 | 10 ± 7 | 2,127 | 3 (1%) | 1.4 | 40 ± 17 | 10 (5%) | 4.7 | 31 ± 11 |
| Aortic coarctation | 157 (17%) | 87 (55%) | 19 ± 7 | 28 ± 9 | 9 ± 6 | 1,406 | 3 (2%) | 2.1 | 35 ± 29 | 7 (4%) | 5 | 28 ± 18 |
| D-transposition | 89 (10%) | 56 (63%) | 19 ± 4 | 28 ± 6 | 9 ± 6 | 838 | 8 (9%) | 9.5 | 26 ± 10 | 10 (11%) | 12 | 26 ± 9 |
| Aortic stenosis | 85 (9%) | 56 (66%) | 19 ± 6 | 30 ± 8 | 11 ± 8 | 971 | 0 | 0 | — | 3 (4%) | 3.1 | 30 ± 4 |
| Pulmonic stenosis | 85 (9%) | 47 (55%) | 22 ± 8 | 30 ± 9 | 8 ± 7 | 719 | 0 | 0 | 20 ± 0 | 2 (2%) | 2.8 | 30 ± 4 |
| Atrioventricular septal defect | 72 (8%) | 27 (37%) | 19 ± 5 | 27 ± 7 | 8 ± 6 | 560 | 1 (1%) | 1.8 | 20 ± 0 | 2 (3%) | 3.6 | 23 ± 4 |
| Ventricular septal defect | 68 (7%) | 30 (44%) | 20 ± 5 | 28 ± 8 | 8 ± 6 | 554 | 2 (3%) | 3.6 | 34 ± 1 | 2 (3%) | 3.6 | 34 ± 1 |
| Atrial septal defect | 57 (6%) | 20 (35%) | 21 ± 7 | 26 ± 8 | 5 ± 5 | 287 | 0 | 0 | — | 0 | 0 | — |
| Single ventricle (Fontan procedure) | 32 (3%) | 14 (44%) | 18 ± 3 | 29 ± 6 | 11 ± 6 | 357 | 1 (3%) | 2.8 | 36 | 5 (16%) | 14 | 25 ± 9 |
| Single ventricle (shunt/Glenn) | 23 (2%) | 10 (44%) | 26 ± 9 | 34 ± 8 | 8 ± 9 | 185 | 1 (4%) | 5.4 | 22 | 3 (13%) | 16 | 32 ± 11 |
| Patent ductus arteriosus | 21 (2%) | 3 (14%) | 28 ± 14 | 36 ± 13 | 8 ± 7 | 159 | 0 | 0 | — | 0 | 0 | — |
| Congenitally corrected transposition of great arteries | 10 (1%) | 6 (60%) | 24 ± 9 | 32 ± 9 | 8 ± 7 | 79 | 2 (20%) | 25 | 39 ± 11 | 5 (50%) | 63 | 31 ± 9 |
| Miscellaneous | 21 (2%) | 10 (48%) | 21 ± 10 | 28 ± 11 | 7 ± 5 | 145 | 1 (5%) | 6.9 | 23 ± 0 | 1 (5%) | 6.9 | 23 ± 0 |
| Overall | 936 | 478 (51%) | 21 ± 7 | 30 ± 8 | 9 ± 7 | 8,387 | 22 (2.3%) | 2.6 | 31 ± 14 | 50 (5.3%) | 5.9 | 29 ± 10 |
| Diagnostic Category | NYHA Class >II | Endocarditis | Reoperation | SVSD | Subaortic Right Ventricle | Clinical Sustained Arrhythmia | Severe Pulmonary Hypertension | SVR | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subaortic | Subpulmonary | Pulmonary | Subpulmonary AV Valve | Subaortic AV Valve | Aorta | |||||||
| Tetralogy of Fallot | 32 (15%) | 8 (4%) | 52 (24%) | 7 (3%) | 34 (16%) | 0 | 35 (16%) | 6 (3%) | 109 (50%) | 25 (12%) | 2 (1%) | 11 (5%) |
| Aortic coarctation | 11 (7%) | 4 (3%) | 18 (11%) | 4 (3%) | 2 (1%) | 0 | 8 (5%) | 10 (6%) | 1 (1%) | 1 (1%) | 5 (3%) | 9 (6%) |
| D-transposition | 11 (12%) | 2 (2%) | 12 (13%) | 28 (31%) | 7 (8%) | 74 (83%) | 37 (42%) | 2 (2%) | 2 (2%) | 4 (4%) | 12 (13%) | 4 (4%) |
| Aortic stenosis | 5 (6%) | 4 (5%) | 13 (15%) | 1 (1%) | 0 | 0 | 4 (5%) | 1 (1%) | 0 | 1 (1%) | 2 (2%) | 23 (27%) |
| Pulmonic stenosis | 9 (11%) | 0 | 13 (15%) | 0 | 8 (9%) | 0 | 9 (11%) | 2 (2%) | 25 (29%) | 8 (9%) | 1 (1%) | 1 (1%) |
| Atrioventricular septal defect | 6 (8%) | 1 (1%) | 6 (8%) | 3 (4%) | 1 (1%) | 0 | 10 (14%) | 5 (7%) | 1 (1%) | 3 (4%) | 18 (25%) | 4 (6%) |
| Ventricular septal defect | 0 | 4 (6%) | 3 (4%) | 2 (3%) | 0 | 0 | 5 (7%) | 1 (1%) | 0 | 1 (1%) | 1 (1%) | 5 (7%) |
| Atrial septal defect | 1 (2%) | 0 | 1 (2%) | 0 | 1 (2%) | 0 | 3 (5%) | 0 | 0 | 0 | 1 (2%) | 0 |
| Single ventricle (Fontan procedure) | 16 (50%) | 0 | 11 (34%) | 6 (19%) | 0 | 3 (9%) | 17 (53%) | 0 | 0 | 0 | 4 (12%) | 0 |
| Single ventricle (shunt/Glenn) | 14 (61%) | 2 (9%) | 8 (35%) | 8 (35%) | 8 (35%) | 4 (17%) | 15 (65%) | 6 (26%) | 2 (9%) | 3 (13%) | 2 (12%) | 0 |
| Patent ductus arteriosus | 1 (5%) | 0 | 2 (10%) | 2 (10%) | 0 | 0 | 1 (5%) | 1 (5%) | 0 | 0 | 0 | 1 (5%) |
| Congenitally corrected transposition of great arteries | 4 (40%) | 0 | 3 (30%) | 7 (70%) | 0 | 10 (100%) | 7 (70%) | 1 (10%) | 0 | 2 (20%) | 5 (50%) | 1 (10%) |
| Miscellaneous | 2 (10%) | 0 | 3 (14%) | 1 (5%) | 1 (5%) | 0 | 7 (33%) | 1 (1%) | 2 (10%) | 3 (14%) | 3 (14%) | 0 |
| Overall | 111 (12%) | 25 (2.7%) | 144 (15%) | 69 (7.4%) | 62 (6.6%) | 91 (10%) | 158 (17%) | 35 (3.7%) | 142 (15%) | 51 (5.4%) | 55 (5.9%) | 59 (6.3%) |
During a total follow-up period of 20 years (8,387 person-years) 50 patients died or underwent transplantation. SCA occurred in 22 patients: 15 patients developed sudden death and 5 patients were successfully resuscitated after SCA and subsequently received an ICD for secondary prevention. In addition, 11 patients underwent ICD implantation for primary prevention (7 patients) or secondary prevention (4 patients). Of these 11 patients, appropriate ICD shocks for ventricular fibrillation were recorded in 2 patients; consistent with our definition of the primary end point, these patients were included in the SCA subgroup. Thus, prevalence and incidence of SCA in the entire sample of repaired CHD were 2.4% and 2.6 per 1,000 person-years, respectively.
In another 22 patients, nonsudden cardiac death occurred from perioperative death (9 patients), heart failure (8 patients), and other cardiovascular causes (5 patients). Nnoncardiovascular death occurred in 2 patients and 4 patients underwent a heart or heart–lung transplantation at follow-up. After excluding aborted sudden death and transplantation, overall mortality was 4.2%, incidence of death was 4.7 per 1,000 person-years, and primary cause of death was SCA (38%) followed by reoperation (23%), heart failure (21%), and other cardiovascular causes (13%). Causes of death by lesions are shown in Figure 1 .
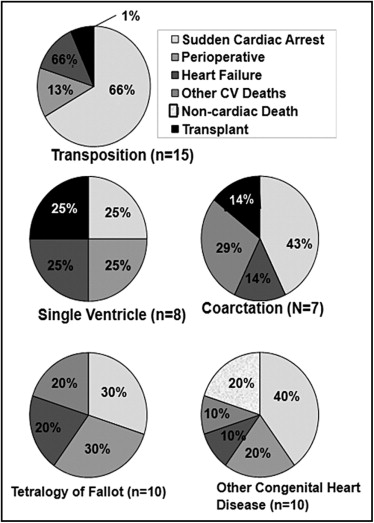
Figure 2 illustrates the incidence of the composite end point of all-cause death or transplantation and of SCA by diagnostic category. Prevalence and incidence varied widely by the specific lesion ( Table 1 ). The highest prevalence of SCA was observed in TGA complexes: 10 of 99 patients (10%) presented with SCA at follow-up, resulting in an incidence of SCA in combined TGA and congenitally corrected TGA of 10 per 1,000 person-years ( Figure 2 ).
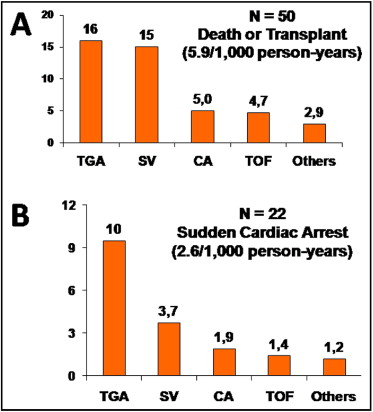
Table 3 presents the proportional distribution of proposed risk factors according to outcomes in the entire cohort. Univariate and multivariate predictors of SCA are listed in Table 4 . Independent factors associated with SCA among the proposed variables were age at first examination and severe subaortic ventricular systolic dysfunction (adjusted hazard ratio 29, 95% confidence interval 11 to 72; Figure 3 ). Comparative data on mortality in patients with severe versus nonsevere subaortic ventricular systolic dysfunction are presented in Table 5 . Interestingly, annual risks of SCA in patients with severely impaired subaortic ventricular systolic function were 2.6% per year in the total CHD population, 3.0% per year for patients with subaortic right ventricular systolic dysfunction, and 3.1% per year for patients with TGA and severely decreased subaortic ventricular systolic function.
| Variables | SCA | All-Cause Death or Transplantation | Survival Free of Transplantation |
|---|---|---|---|
| (n = 22) | (n = 50) | (n = 886) | |
| Age at first examination (years) | 24 ± 14 | 22 ± 9 | 21 ± 7 |
| Men | 15 (68%) | 28 (56%) | 450 (51%) |
| New York Heart Association class >II (%) | 7 (32%) † | 27 (54%) ⁎ | 84 (9%) |
| Left ventricular outflow tract obstruction | 3 (14%) | 10 (20%) | 232 (26%) |
| Tetralogy of Fallot | 3 (14%) | 10 (20%) | 206 (23%) |
| Systemic-to-pulmonary shunt | 3 (14%) | 4 (8%) † | 214 (24%) |
| Transposition of great arteries | 10 (45%) ⁎ | 15 (30%) ⁎ | 84 (9%) |
| Single-ventricle physiology | 2 (9%) | 8 (16%) † | 47 (5%) |
| Severe subaortic ventricular systolic dysfunction | 16 (73%) ⁎ | 28 (56%) ⁎ | 41 (5%) |
| Severe subpulmonary ventricular systolic dysfunction | 5 (23%) † | 12 (24%) ⁎ | 50 (6%) |
| Morphologic subaortic right ventricle | 9 (41%) ⁎ | 14 (28%) ⁎ | 77 (9%) |
| Clinical sustained arrhythmias | 14 (64%) ⁎ | 23 (46%) ⁎ | 135 (15%) |
| Severe pulmonary hypertension | 3 (14%) † | 10 (20%) ⁎ | 25 (3%) |
| Severe pulmonary valve regurgitation | 3 (14%) | 8 (16%) | 134 (15%) |
| Severe subpulmonary atrioventricular valve regurgitation | 2 (9%) | 3 (6%) | 48 (5%) |
| Severe subaortic atrioventricular valve regurgitation | 3 (14%) | 9 (18%) ⁎ | 47 (5%) |
| Severe aortic valve regurgitation | 0 | 4 (8%) | 55 (6%) |
| Infective endocarditis | 0 | 2 (4%) | 23 (3%) |
| Need for reintervention | 5 (23%) | 21 (42%) ⁎ | 123 (14%) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


