The five adult clinical cluster phenotypes from the Severe Asthma Research Program (SARP) with the predominant clinical characteristics. (Reprinted with permission of the American Thoracic Society. Copyright © 2019 American Thoracic Society. From: Jarjour et al. [25]. The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society)
Two follow-up cluster analyses on the adult subjects in SARP incorporated biomarkers as variables (“inflammatory clustering”) [53, 54]. The first analysis applied a machine learning to 378 SARP subjects using >100 clinical variables, exhaled nitric oxide (FeNO) measurements, and inflammatory cell counts from blood and bronchoalveolar lavage [53]. Of the six phenotypes identified, five were similar to the initial clinical clusters reported in SARP with an additional phenotype of men with adult onset asthma and prominent nasal polyposis that has been described in other cohorts. The second SARP inflammatory cluster analysis included both blood and sputum granulocyte counts with the most discriminating 11 variables from the original cluster analysis and identified four clusters that again resemble the initial clinical cluster phenotypes [54]. Blood and sputum eosinophilia were seen in some subjects in all clusters and were not specific for a given phenotype. Sputum neutrophils were seen predominantly in the two severe clusters, and the majority of the most severe group had concurrent elevations in both eosinophils and neutrophils. Discriminant analysis identified sputum neutrophilia and pre-bronchodilator FEV1% predicted to be the most influential variables in this inflammatory cluster, supporting an association with poor lung function, T2-low pathobiologic mechanisms, and disease severity.
SARP Pediatric Cluster Analysis
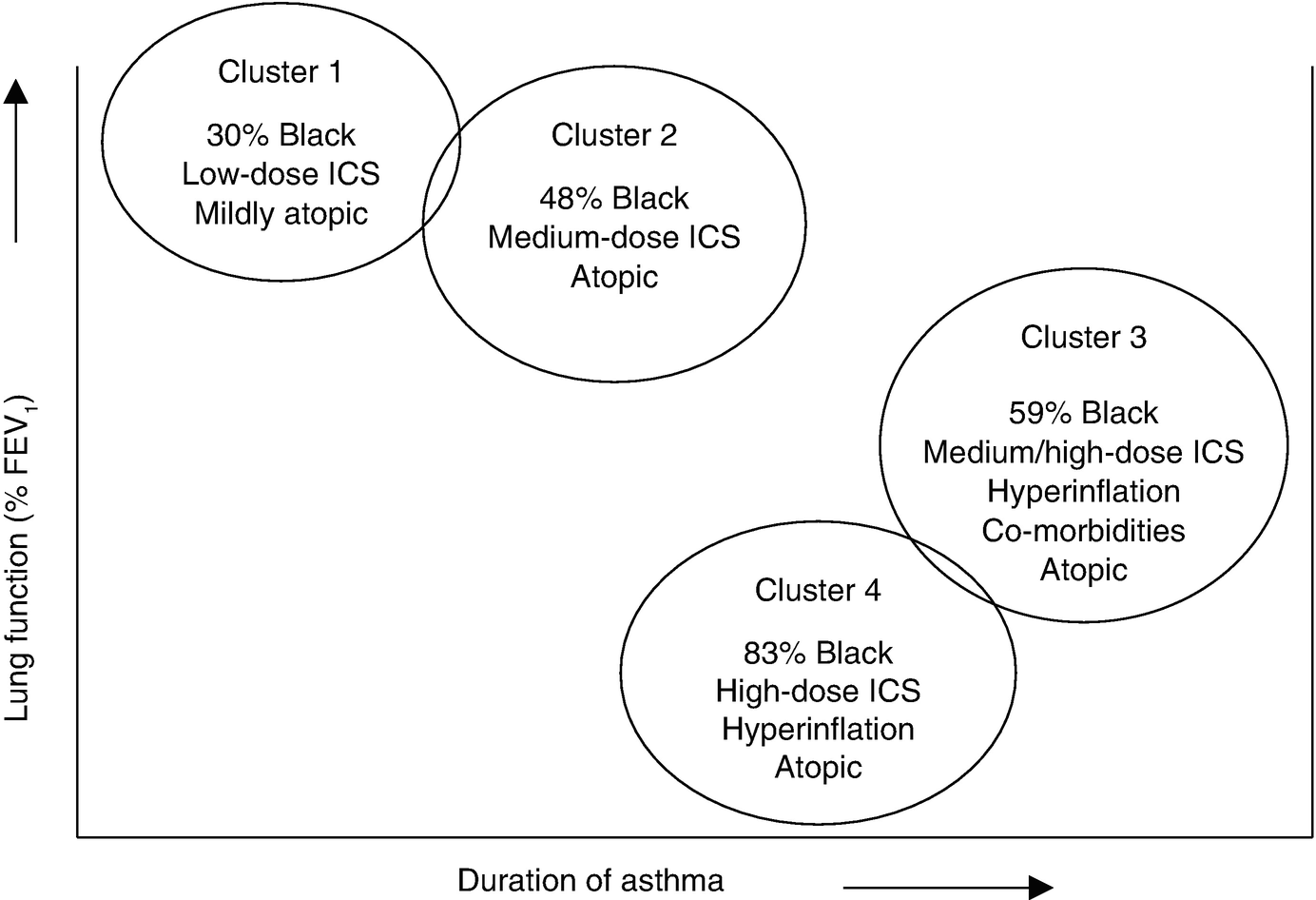
The four pediatric clinical cluster phenotypes from the Severe Asthma Research. Program (SARP) . (Reprinted with permission From: Fitzpatrick et al. [55])
Coordinating Cluster Phenotypes
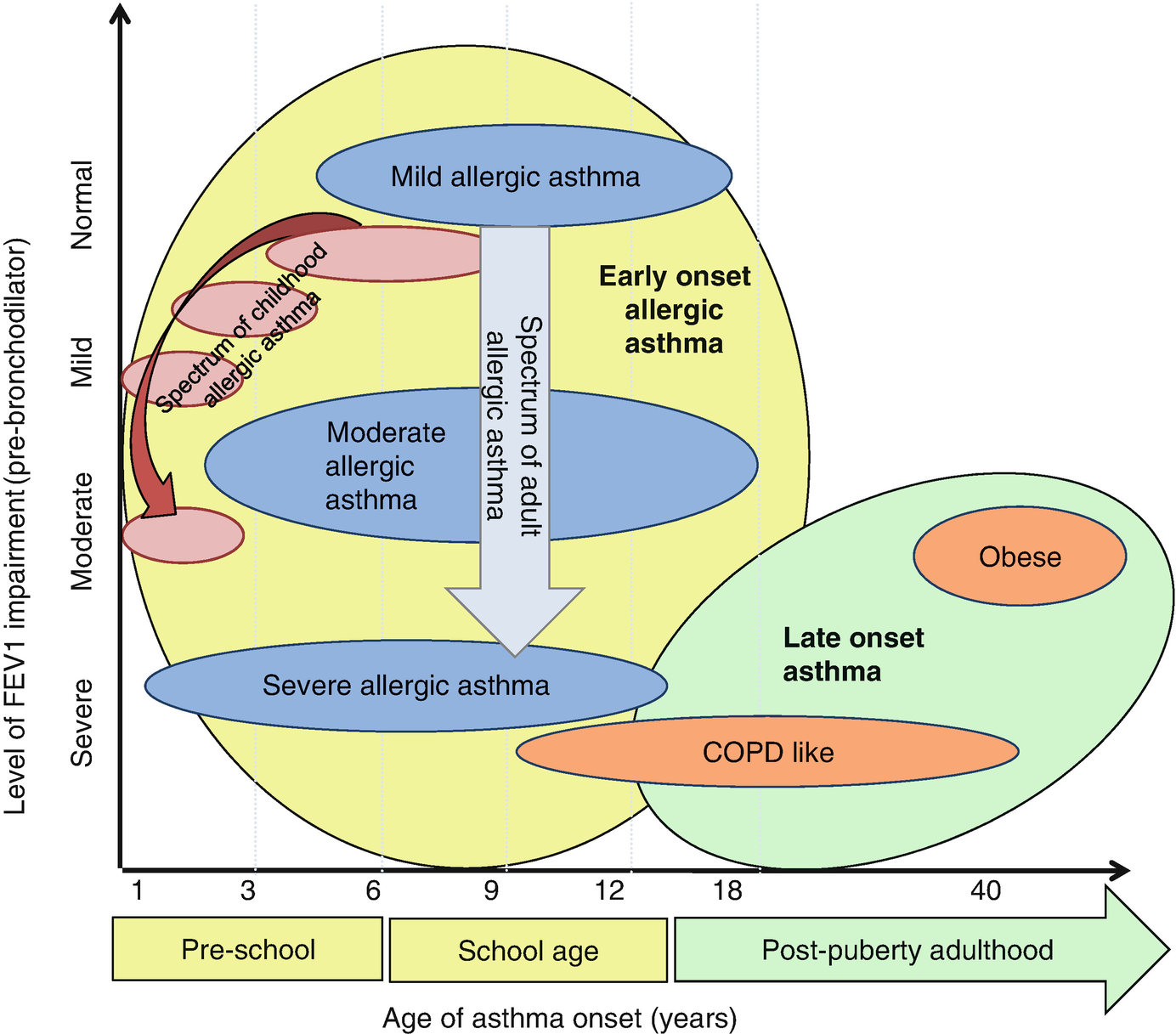
A proposed combination of the adult and pediatric SARP clinical clusters. Generally, earlier age of asthma onset in childhood appears to be associated with progressively more severe allergic asthma in both children and adults. Heterogeneity within each phenotype is indicated by the shape of the oval; the “width” represents heterogeneity in the age of asthma onset, the “height” symbolizes heterogeneity of lung function within each group. (Reprinted with permission of the American Thoracic Society. Copyright © 2019 American Thoracic Society. From: Moore et al. [59]. The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society)
All five SARP adult clinical cluster phenotypes are reproducible in the newest cohort of (SARP3) which includes longitudinal follow-up of 700 asthma subjects (60% severe by ATS/ERS definition). In addition, these five phenotypes have also been replicated in “real life” clinics and other cohorts using a three-variable algorithm that incorporates pre- and post-bronchodilator % predicted FEV1 and age of asthma onset to “classify” individual patients [60–63].
Nearly all the published cluster analyses have used cross-sectional data to identify what are essentially “static” phenotypes because our knowledge of the natural history of these phenotypes is limited. Several studies have reported that nearly half of adult patients demonstrate cluster stability over time, although the severe asthma cluster phenotypes are somewhat less stable temporally [46, 47, 50, 52, 62, 63]. The SARP3 cohort will allow assessment of the stability of the original SARP pediatric and adult cluster phenotypes over time as well as investigation into the effect of cluster phenotypes on outcomes associated with severe asthma such as progressive lung function decline and asthma exacerbations [64].
7.6 Accepted Severe Asthma Phenotypes to Use in Clinic Today
Taken together, “biased, hypothesis-driven” and “unbiased, multivariate” approaches have led to several generally accepted severe asthma phenotypes that are distinct and representative of the patients seen in clinical practice [65]. These phenotypes incorporate clinical traits and biomarkers that will empower the use of phenotype-driven therapeutic approaches in clinical practice by associating what we can “see” in clinic and what therapy (above the usual inhaled medications) patients should receive as “add-on” therapy. There are two severe asthma phenotypes (both T2-high phenotypes) that are best defined and ready for use in clinic to identify patients who may benefit from the T2-high biologic immunomodulators on the market today.
Childhood (Early) Onset Severe Allergic Asthma
Traditional “allergic ” asthma is the most common phenotype in severe asthmatic children and in many ways the most “recognizable” severe phenotype in adults. Atopy and allergic co-morbidities are the hallmark of allergic asthma, and they are more intense in the children than the adults. Elevations in serum IgE, blood eosinophils and exhaled nitric oxide (FeNO) are common suggesting that the underlying pathobiologic mechanism in this group is driven by Th2 lymphocyte adaptive immunity or “T2-high” inflammation [66]. Both SARP children and adults with severe allergic asthma (Cluster 4) had evidence of T2-high inflammation and clinically “labile” long duration disease with frequent exacerbations. Immunomodulators such as omalizumab (anti-IgE) and dupilumab (anti-IL4 receptor) are designed to alter pathways important in atopy and have been shown to reduce asthma exacerbations by 50–75% in patients with clinical characteristics and biomarker patterns similar to SARP Cluster 4 phenotype [67–70].
Adult Onset Severe Eosinophilic Asthma
Eosinophilic asthma is among the best defined adult asthma phenotypes. This group of patients largely exists in allergy and otolaryngology clinics struggling with chronic rhinosinusitis, with or without nasal polyps and aspirin sensitivity, or subspecialty severe asthma clinics where many are dependent on chronic oral corticosteroids [44, 71]. These patients are typically not atopic or have only limited atopy without the clinical relevance seen in allergic asthma. In the SARP cluster analysis these patients resided in Cluster 3 and the “older” onset patients in Cluster 5. Blood and/or sputum eosinophilia is the hallmark of eosinophilic asthma. Many of these patients have a dramatic response to high doses of systemic corticosteroids (particularly the nasal symptoms) but cannot be controlled with high-dose inhaled steroids alone. Three immunomodulators (mepolizumab, reslizumab, benralizumab) that downregulate eosinophilia (anti-IL5 or anti-IL5 receptor) have been shown to decrease exacerbations rates in this group of patients by 50–75% when patients with the appropriate phenotype are selected [72–77].
7.7 Summary: Severe Asthma(s)
Asthma is a heterogeneous disorder manifested by common symptoms of reversible airflow obstruction. Severe asthma represents a minority of the patients with asthma but accounts for most of the health-care expenses associated with this disease. Novel therapies are being developed to target the underlying inflammation and also the treatable underlying traits of an individual patient. Approaches to therapeutic management that do not take into account the underlying heterogeneity in the clinical phenotype are likely to fail due to lack of specificity.
Using both hypothesis-driven and unbiased multivariate analyses, several severe asthma phenotypes have been identified and these are being refined on an ongoing basis. While some of these phenotypes are ready to be used in clinical practice to guide targeted therapy (severe childhood-onset atopic asthma or adult-onset eosinophilic asthma), the fact that not all patients respond to these therapies exemplifies that there is much “fine tuning” to do before this is a fail proof system to guide therapy. Nearly all of the described phenotypes were identified in cross-sectional cohorts but we know much less about longitudinal stability of these phenotypes, especially the relationship between pediatric and adult phenotypes. We also do not know the ability of severe asthma phenotypes to predict long-term outcomes of disease; these studies are underway in severe asthma cohorts across the globe to better direct therapy in the future.

Full access? Get Clinical Tree


