Fig. 1
Open field test in rats. The yellow tangled lines show the trajectory of movement of a healthy untreated rat during 15 min of the test. The inset in the upper right corner shows the rat immobilization in the reserpine model of parkinsonism
2.2 Ventilation Measurements
Ventilation and its poikilocapnic responses to hypoxia were investigated in a whole-body flow-through plethysmograph (model PLY3223; Buxco Electronics, Wilmington, NC). Rats remained unrestricted in the recording chamber. As soon as the rat familiarized with the chamber environment (about 30–40 min) basal ventilation was recorded in room air. Acute hypoxia was achieved by a rapid flushing of air balanced with N2. Two levels of the hypoxic stimulus were applied in a random manner: 12 % and 8 % O2 in N2. The gas equilibration time in the chamber took 45–60 s from a gas switch, after which time the recording started. Pressure difference between the recording and reference chambers was measured with a differential pressure transducer. The breath-by-breath signal was integrated and further processed by data analysis software (Biosystem XA for Windows SFT3410 ver. 2.9; Buxco Electronics, Wilmington, NC), yielding instantaneous minute ventilation (VE, ml∙min−1, BTPS), as the product of tidal volume (V T , ml) and breathing frequency (f, breaths∙min−1). The hypoxic tests were of 3 min duration, with a 10–15 min recovery interval of room air breathing between the tests. A bias flow at a rate of 2.5 l/min was employed between the tests to remove the CO2 build-up from the chamber. Data were tallied off-line at 30 s time marks of the hypoxic course; each variable was expressed as an average of the 10 s bin preceding the time mark.
2.3 Statistical Elaboration
Data were expressed as means ± SE. Variables were normalized to kg of body weight. The analysis focused on three main points of interest characterizing ventilatory changes: prehypoxic basal control (0 s point), peak hypoxic response (30 s point), and the end-test hypoxic fall-off (180 s point). These three main time marks of the HVR were compared with one-way ANOVA with repeated measures, followed by the post hoc Tukey test if significant changes were detected. A two-tailed paired t-test was used for comparison of ventilatory data at the corresponding time points. Finally, two-way ANOVA with repeated measures in time factor was applied to compare time courses of HVRs in various pharmacological conditions employed (second factor). P < 0.05 was used as a definer of statistical significance.
3 Results
3.1 Ventilation in Parkinsonism
The HVR had a classical biphasic stimulatory/inhibitory character in both healthy untreated and parkinsonic reserpine-treated rats. The peak VE response developed promptly within 30 s from hypoxia commencement followed by a gradual fall-off; with ventilation remaining above the resting baseline level within the test time. Peak ventilatory augmentation at both hypoxic levels employed, as shown in all relevant figures below, was invariably significant (0.05 > p > 0.0001). Parkinsonic VE was significantly dampened across the course of the hypoxic course compared with that in the healthy group (Fig. 2a, b). The dampening was present in response to both 12 % and 8 % hypoxia and was notably expressed in relation to the peak response at 30 s. The ventilatory response to the stronger 8 % hypoxic stimulus expectedly demonstrated a more conspicuous hyperpneic stimulation as well as reserpine-induced inhibition; where the mean peak VE decreased from 1644 ± 82 ml∙min−1∙kg−1 in the healthy to 1264 ± 73 ml∙min−1∙kg−1 in the parkinsonic rats (p < 0.0001) (Fig. 2b). The parkinsonic condition also decreased the baseline resting VE before the initiation of hypoxia. This decrease, albeit significant, was disproportionally smaller than that of peak VE. Therefore, the dampening ventilatory effect of the parkinsonic condition was even more noticeable in the augmentation of VE from baseline to peak amounting to 771 ± 52 and 532 ± 47 ml∙min−1∙kg−1 (p < 0.01) in the healthy and parkinsonic rats, respectively, in response to 8 % hypoxia. The three null hypotheses tested by two-way ANOVA that time has no effect on the ventilatory outcome, reserpine treatment makes no ventilatory difference, and there is no interaction between time and treatment regarding the ventilatory outcome were disproved (p < 0.001) (Fig. 2b). No differences between the effects of reserpine and reserpine + DOM were detected in the VE responses to either 12 % or 8 % hypoxia.
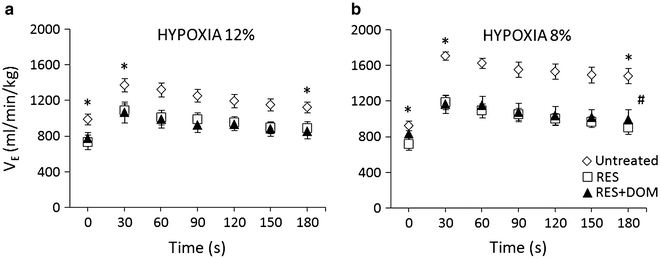

Fig. 2
Ventilatory responses to acute 12 % and 8 % hypoxia in the healthy untreated condition (open diamond-shaped symbols), in reserpine-induced parkinsonism (open squares), and in domperidone (DOM)-treated reserpinized rats (solid triangles). The rats were their own controls (n = 8). Parkinsonic condition caused an overall decrease in ventilation and its responses to hypoxia. (a) – *p < 0.02 for the decreases in ventilation in reserpine-treated and reserpine + DOM-treated vs. untreated rats. at 0, 30, and 180 s time points; (b) – *p < 0.001 for the difference between ventilation in reserpine-treated vs. untreated rats at 0 s, and *p < 0.0001 between reserpine-treated and reserpine + DOM-treated vs. untreated rats at 30 s and 180 s; #p < 0.001 for the effects of type of treatment, time, and the interaction of treatment with time
3.2 Peripheral and Central Dopamine Modulation of Hypoxic Ventilation in Parkinsonism
Domperidone, a peripheral antagonist of DA D2 receptors which does not cross the blood-brain barrier, given on top of reserpine failed to appreciably influence baseline ventilation and its responses to hypoxia (Fig. 2a, b). That was in contrast to a waxing effect of domperidone on the HVR in the untreated healthy rats, which was particularly expressed during the stronger 8 % hypoxic stimulation (Fig. 3b).
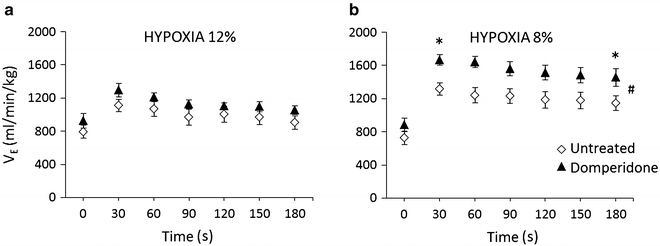

Fig. 3
Influence of domperidone (solid triangles) on resting and hypoxic ventilation in control untreated rats (open diamond-shaped symbols) (n = 7). (a) – 12 % hypoxia; (b) – 8 % hypoxia, *p < 0.03 for the difference between ventilation in domperidone-treated vs. untreated rats at 30 s and 180 s; #p < 0.001 for the effects of time, type of treatment, and the interaction of treatment with time
Conversely, L-DOPA with benserazide, the latter inhibits peripheral L-DOPA metabolism which enables it to cross the blood-brain barrier in full, a central DA D2 receptor agonist, given on top of reserpine significantly enhanced reserpine-dampened resting ventilation and its responses in both mild and strong hypoxia (Fig. 4a, b). The enhancement exceeded, although insignificantly, the baseline ventilatory level over the course of the hypoxic responses, yielding a stimulatory response overshoot. The peak ventilatory responses to the stronger 8 % hypoxic stimulus at 30 s were as follows: baseline in the untreated rats of 1409 ± 62 ml∙min−1∙kg−1 decreased to 1017 ± 117 ml∙min−1∙kg−1 in reserpine-induced parkinsonism, and rebounded to 1661 ± 168 ml∙min−1∙kg−1 after giving L-DOPA with benserazide on top of reserpine; differences in VE between reserpine-treated vs. untreated and reserpine + L-DOPA-treated rats were significant (p < 0.01) (Fig. 4b). There were significant treatment (p < 0.04) and time (p < 0.001) effects on the ventilatory outcome, and an interaction between the two (p < 0.001) regarding the HVR after reserpine vs. reserpine + L-DOPA for 12 % and 8 % hypoxia and the HVR in untreated vs. reserpine-treated rats for 8 % hypoxia (two-way ANOVA).
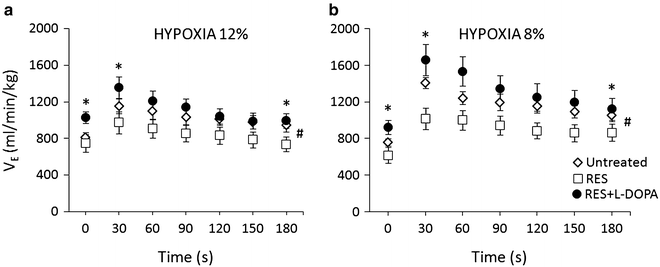

Fig. 4
Ventilatory responses to acute 12 % and 8 % hypoxia in the healthy untreated condition (open diamond-shaped symbols), in reserpine-induced parkinsonism (open squares), and in L-DOPA + benserazide-treated reserpinized rats (solid circles). The rats were their own controls (n = 7). Parkinsonic condition caused an overall decrease in ventilation and its responses to hypoxia and L-DOPA caused ventilation to rebound beyond the level present in healthy untreated rats. (a) – *p < 0.005 for the differences in ventilation between reserpine + L-DOPA-treated vs. untreated and reserpine-treated rats at zero time point and *p < 0.002 for differences between reserpine + L-DOPA-treated vs. reserpine-treated rats at 30 and 180 s; (b) – *p < 0.01 for the differences between reserpine-treated vs. reserpine + L-DOPA-treated rats at 0 s, reserpine-treated vs. untreated and reserpine + L-DOPA-treated rats at 30 s, and reserpine-treated vs. reserpine + L-DOPA-treated rats at 180 s; #significant effects of type of treatment (p < 0.04), time and the interaction of treatment with time (p < 0.001) for reserpine vs. reserpine + L-DOPA (panels a and b) and for untreated vs. reserpine-treated rats (panel b)
L-DOPA with benserazide given to otherwise untreated rats, showed just a tendency to enhance ventilation and its responses to both 12 % and 8 % hypoxia, which failed to reach significance across the response course (Fig. 5).
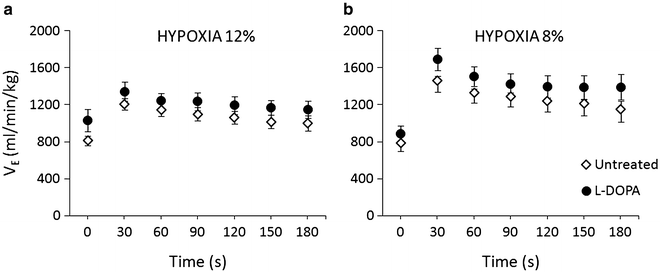

Fig. 5
Influence of L-DOPA (solid circles) on resting and hypoxic ventilation in healthy untreated rats (open diamond-shaped symbols) (n = 5). (a) – 12 % hypoxia; (b) – 8 % hypoxia
The inspection of the breathing pattern shows that the frequency component contributed more to shaping the ventilatory changes. Breathing rate decreased by about 30–40 % in reserpinized rats along the HVR. This decrease tended to be compensated by increases in tidal component, which were disproportionately smaller and thus fell short of offsetting the overall decrease in VE (Table 1). Domperidone failed to affect the breathing patter. L-DOPA, on the other hand, increased both components, in particular breathing rate reverted, on average, about halfway toward the baseline level present in the healthy untreated condition.
Table 1
Contribution of frequency (f) and tidal (VT) components to hypoxic ventilatory changes at baseline (0 s), peak (30 s), and nadir (180 s) of response in the reserpine (RES) model of parkinsonism
Condition | f (breaths∙min−1) | VT (ml∙kg−1) | ||||
|---|---|---|---|---|---|---|
0 s | 30 s | 180 s | 0 s | 30 s < div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||