Importance of Left Ventricular Diastolic Function and Prediction of Clinical Outcomes Following Cardiac Resynchronization Therapy
Alan D. Waggoner
Lisa de las Fuentes
Victor G. Davila-Roman
Diastolic function is invariably impaired in patients with symptomatic heart failure (HF). In those with HF secondary to systolic dysfunction, left ventricular (LV) diastolic dysfunction is a major contributor to symptoms of heart failure and has been reported to predict longterm outcomes. Although cardiac resynchronization therapy (CRT) is directed at improvement in LV dyssynchrony and systolic function, its effects on diastolic function and whether diastolic function may be a predictor of clinical outcomes has been less well-characterized.1,2,3 This chapter will review the results of studies that have evaluated LV diastolic function for patients treated with CRT immediately after device implant, and at early and intermediate follow-up after CRT. Furthermore, the authors discuss indices of LV diastolic function that predict clinical outcomes after CRT.
Diastolic Function in Heart Failure
Abnormalities in diastolic function are known to play a major role in symptoms of heart failure.4 For example, increases in preload (i.e., increased LV end-diastolic volume and/or end-diastolic pressures) result in an upward shift of the diastolic pressure/volume relationship with reduced LV compliance (i.e., a “stiff” left ventricle). Decreased LV compliance in systolic heart failure is often due to myocardial fibrosis (i.e., a result of long-standing hypertension and hypertrophy) and/or myocardial scarring (resulting from prior myocardial infarction). Increased LV end-systolic volume alters elastic recoil and results in prolonged LV relaxation. Increased afterload (i.e., increased blood pressure and/or increased wall stress) due to LV dilatation, particularly in the absence of a compensatory increase in wall thickness (i.e., eccentric LV hypertrophy), may cause subendocardial ischemia, and the combination results in decreased LV contractility and prolongation of LV relaxation. Two additional issues play an important role in the systolic/diastolic interactions in candidates for CRT. First, the presence of abnormal ventricular conduction (i.e., left bundle branch block) causes discordant LV contractility, which further exacerbates LV systolic and diastolic performance. Second, increased left atrial volumes and impaired left atrial function play an important role in compromising diastolic function.5
Stages of LV Diastolic Dysfunction
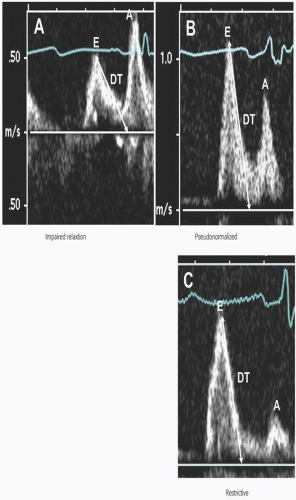
One approach for staging the severity of LV diastolic dysfunction in HF patients with LV systolic dysfunction is based on patterns of LV diastolic filling determined by pulsed-wave Doppler mitral inflow velocities, as shown in Figure 3.1. It is important to recognize that mitral inflow velocities are influenced by the combined effects of changes in LV relaxation, instantaneous changes in left atrial and LV diastolic pressures, and LV compliance.6,7 Impaired relaxation is universally present in HF patients. Early diastolic LV filling pressures may be relatively normal in some patients with LV systolic dysfunction, but LV end-diastolic pressure is usually elevated. The pulsed-wave Doppler mitral inflow pattern of impaired relaxation in this setting demonstrates a low mitral E-wave velocity and E/A ratio, and prolonged deceleration time (DT). An intermediate stage of LV diastolic dysfunction, described as a “pseudonormalized pattern,” occurs when early LV filling pressures are elevated and increases mitral E-wave velocity and the E/A ratio with normalization or shortening of DT. LV relaxation, however, typically remains abnormally prolonged. Restrictive LV filling in HF patients is a result of markedly increased LV filling pressures and the LVEDP. The mitral E-wave velocity and E/A ratio are increased, the A-wave velocity is diminished, and the mitral DT is shortened. Several studies have reported that these patterns of pulsed-wave Doppler-derived mitral inflow velocities predict clinical outcomes in patients with HF and LV systolic dysfunction.8,9,10,11,12,13 The cumulative results of these studies indicate that a restrictive LV filling pattern is associated with higher morbidity and mortality, regardless of the impairment in LV systolic dysfunction (determined by the LV ejection fraction), compared
to patients with an impaired relaxation, or a “pseudonormalized” filling pattern.
to patients with an impaired relaxation, or a “pseudonormalized” filling pattern.
Assessment by Tissue Doppler Imaging
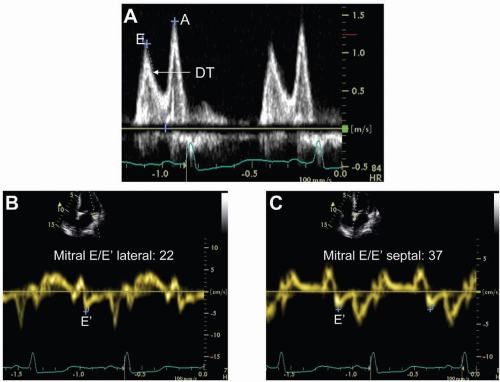
Another method for assessing LV diastolic function includes tissue Doppler imaging (TDI) measurements of early diastolic mitral annular velocities (E’) that are a relatively load-independent determinant of LV relaxation.14 TDI measurements of E’ are obtained by placement of a sample volume in the myocardial wall, adjacent or superior to the mitral annulus, in the apical four-chamber and two-chamber views as shown in Figure 3.2. Impaired LV relaxation results in a decrease in the E’ velocity but is relatively unaffected by the changes in early LV filling pressure. Importantly, the ratio of the mitral E-wave/E’ velocity at the lateral and/or at the
septal annulus can be used to accurately estimate LV filling pressures.15,16 As early LV filling pressures increase, with an accompanying increase in mitral E-wave velocity, E’ remains low due to the underlying myocardial relaxation abnormality. One study reported that a reduced E’ and a mitral E/E’ ratio >20 provides incremental value to predict cardiac related events, in addition to mitral E-wave deceleration time.17
septal annulus can be used to accurately estimate LV filling pressures.15,16 As early LV filling pressures increase, with an accompanying increase in mitral E-wave velocity, E’ remains low due to the underlying myocardial relaxation abnormality. One study reported that a reduced E’ and a mitral E/E’ ratio >20 provides incremental value to predict cardiac related events, in addition to mitral E-wave deceleration time.17
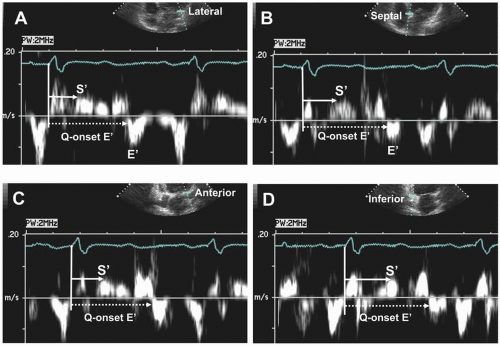
TDI-derived measurements of the timing of E’ have been used to determine LV diastolic dyssynchrony in HF patients and the response to CRT.18,19,20 Diastolic dyssynchrony is determined by measurements of the time from onset of the QRS to the onset, or to peak E’ velocities, at several mitral annular sites (Fig. 3.3). The extent of diastolic dyssynchrony is expressed as the maximal difference of the time intervals between sites in the apical views. The maximal difference between individual LV segments in normal subjects is <40 ms.19
Acute Effects of CRT on LV Diastolic Function
Invasive studies performed at the time of device implant have shown variable effects of CRT on LV diastolic function. In separate single-center studies that included 41 patients (32% ischemic), pulmonary artery capillary wedge pressure and/or LV end-diastolic pressure (LVEDP) immediately after CRT decreased or remained relatively unchanged.21,22 Another single center study reported that, in subjects with a high LVEDP, significant decreases can occur immediately after CRT; conversely, if LVEDP is normal, minimal or no changes are observed.23 The programmed atrioventricular (AV) delay during CRT also affects LV diastolic function.24,25 While relatively short programmed AV delays (i.e., <100 ms) decrease LVEDP, this reduction may occur at the expense of decreased stroke volume and cardiac output.24, 26 These findings may be
explained by the fact that patients with HF due to LV systolic dysfunction require increased LV filling pressures for optimal systolic performance.1 Invasive studies have also shown that, despite significant increases in peak-positive LV dP/dt immediately after CRT, these are not accompanied by significant changes in LV relaxation, determined by peak-negative LV dP/dt or Tau (the time constant of LV relaxation).27,28,29 However, left ventricular diastolic filling time usually increases during CRT, regardless of changes in LV filling pressures or relaxation, as a result of improved atrio-ventricular (AV) synchrony with programming the AV delay of the CRT device.
explained by the fact that patients with HF due to LV systolic dysfunction require increased LV filling pressures for optimal systolic performance.1 Invasive studies have also shown that, despite significant increases in peak-positive LV dP/dt immediately after CRT, these are not accompanied by significant changes in LV relaxation, determined by peak-negative LV dP/dt or Tau (the time constant of LV relaxation).27,28,29 However, left ventricular diastolic filling time usually increases during CRT, regardless of changes in LV filling pressures or relaxation, as a result of improved atrio-ventricular (AV) synchrony with programming the AV delay of the CRT device.
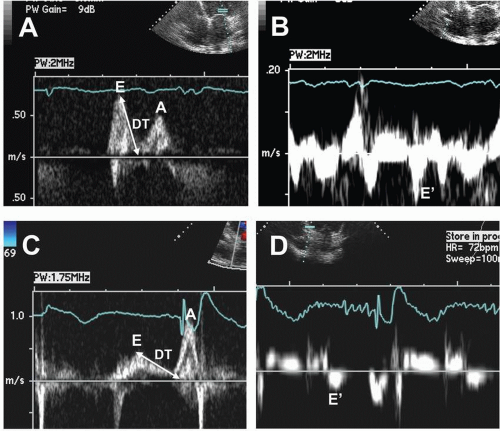
Two-dimensional and Doppler echocardiography has also been used to assess the acute effects of CRT on measurements of systolic and diastolic function.30,31,32 Although significant reductions in LV volumes and increases in LV ejection fraction (LVEF) occur immediately after CRT, the responses in PWDand TDI-derived indices of diastolic function may depend on the pre-CRT diastolic filling pattern. In patients with a pattern of impaired LV relaxation, CRT did not result in significant changes in PWD-derived E-wave velocity, A-wave velocity, the E/A ratio, or the deceleration time, although diastolic filling time increased significantly.33 TDI-derived E’ velocities remain unchanged or may actually decrease; the E/E’ ratio also remains unchanged.31,32 In patients with pseudonormalized or restrictive LV filling patterns, however, the PWD-derived E-wave velocity decreases, DT increases, and the E/E’ ratio decreases, suggesting reduction in LV filling pressures (Fig 3.4). Thus, the results obtained by two-dimensional and Doppler echocardiography, immediately after CRT, are similar to those reported by invasive measurements. It is not known whether the acute response in left ventricular diastolic function immediately after CRT predicts functional improvement and/or clinical outcomes during long-term follow-up.
Effects of CRT on LV Diastolic Function at Early Follow-Up
Invasive studies of diastolic function at 3 to 6 months following CRT are limited. One study of 22 patients (64% ischemic) reported that the LVEDP decreased and peak-negative
LV dP/dt increased, although Tau remained unchanged at 6-month follow-up.34 Significant reductions in LV enddiastolic volume after CRT were observed and may have influenced the response in both LVEDP and peak-negative LV dP/dt, which are preload-dependent measurements.35
LV dP/dt increased, although Tau remained unchanged at 6-month follow-up.34 Significant reductions in LV enddiastolic volume after CRT were observed and may have influenced the response in both LVEDP and peak-negative LV dP/dt, which are preload-dependent measurements.35
Results of early follow-up studies after CRT assessed by two-dimensional and Doppler echocardiography have shown variable findings (Table 3.1). LV diastolic filling time invariably increases at early follow-up after CRT.36,37,38,39,40,41,42,43,44,45 In a large CRT multicenter clinical trial of 172 patients, LV diastolic function was evaluated by PWD-derived measurements at baseline and at 3 months, and 6 months after CRT. There were significant decreases in mitral E-wave velocity and increase in DT and DFT, but the mitral E/A ratio remained unchanged from pre-CRT levels.37 The results demonstrated that CRT had a beneficial effect on diastolic function, manifested by improved LV diastolic filling and/or decreased LV filling pressures (i.e., decreased E and increased DT). Significant improvement in LVEF and decrease in LV volumes in the CRT group were observed, although evident to a greater extent in the nonischemic group. It was not reported, however, whether HF etiology (i.e., ischemic vs. nonischemic) was a factor in the improvement in LV diastolic filling indices. Thus, the results suggest that improvements in LV diastolic function after CRT are coupled to the beneficial effects on systolic performance and/or reverse remodeling.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree