The implantable cardioverter defibrillator (ICD) is a device that can recognise and automatically terminate ventricular tachycardia or fibrillation by delivering an appropriate electrical therapy.
The therapies are:
In addition, the device can act as a pacemaker to prevent bradycardia and to provide a chronotropic response to exercise.
The first defibrillator was implanted in 1980. Early devices were very large, necessitating implantation abdominally in the rectus sheath, and employed epicardial leads.
Current devices are somewhat larger than a pacemaker and are usually implanted subcutaneously over pectoralis major (in thin patients the device can be implanted beneath the pectoral muscle). Therapy is delivered via a right ventricular lead introduced via the cephalic or subclavian vein. Like a pacing lead, there is an electrode at its tip for pacing and sensing. Just proximal to the tip is a coil that acts as the cathode so a DC shock can be delivered between it and the device case or ‘can’, which is the anode. ‘With ‘dual-coil’ leads there is an additional coil located a few centimetres more proximally so that it lies in the superior vena cava. It also acts as an anode together with the can. In theory, a dual-coil lead may enable lower energy requirements for shock delivery, but most studies show no significant advantage of dual-coil as compared with single-coil leads.
In patients without persistent atrial fibrillation, dual-chamber systems are often employed, requiring the addition of an atrial pacing lead. Dual-chamber devices provide the same benefits as dual-chamber pacemakers for the management of bradycardia. Additionally, atrial sensing enables better discrimination between supraventricular and ventricular arrhythmias.
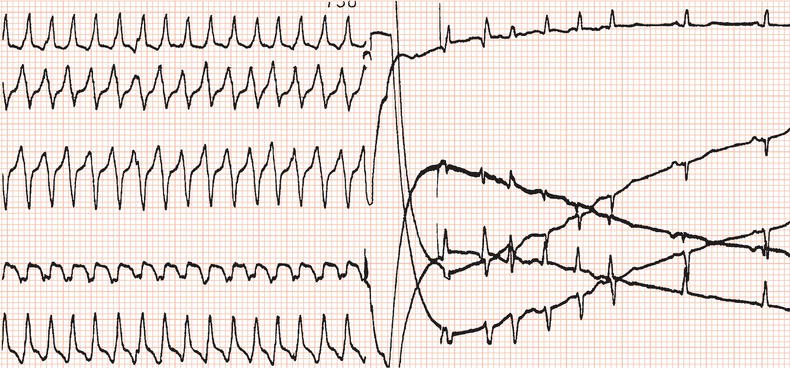
Figure 24.3 Initiation of ventricular fibrillation by delivering a low-energy DC shock on the T wave of the eighth paced beat.
Defibrillator implantation
The implantation procedure for a transvenous system is similar to that for a pacemaker and is usually carried out under local anaesthesia. The risks of complication are the same as for pacemaker implantation. Infection and hematoma are the commonest: these are also not infrequently encountered at ICD generator replacement.
Defibrillation threshold
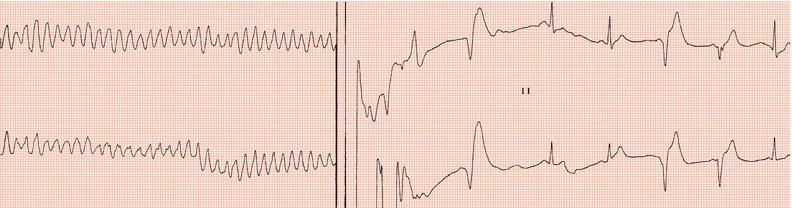
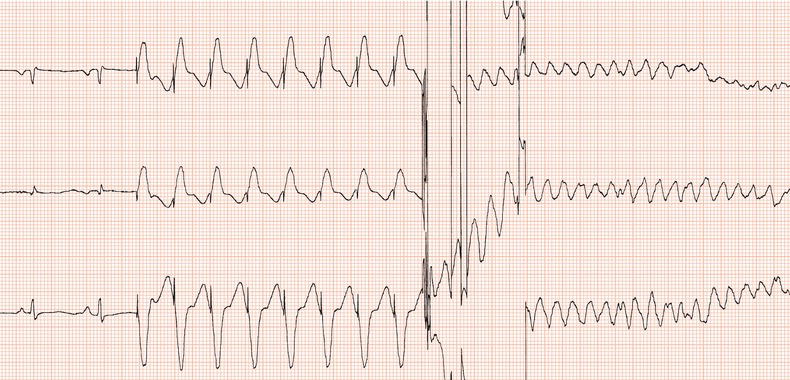
Routine practice was to ensure that the device could terminate ventricular fibrillation by measuring the defibrillation threshold (DFT): after hefty intravenous sedation, ventricular fibrillation was initiated by delivering via the implanted device a DC shock coincident with the T wave (Figure 24.3), or by a burst of alternating current (50 or 60 Hz), or by a brief, very rapid train of ventricular stimuli. It was desirable to establish that an output at least 10 J less than the device’s maximum output, which is in the region of 35 J, was effective. Usually, the initial output would be set to 18 J. The device was programmed to deliver a 24 J shock if the first one failed: if the second shock failed, immediate external defibrillation would be carried out. It was interesting that sometimes ventricular fibrillation terminated spontaneously while the device was charging! If a high DFT was encountered then sometimes programming reversal of polarity such that the distal coil acted as the anode could lead to a lower DFT; otherwise lead repositioning was required.
However, with modern devices the DFT is almost always found to be satisfactory, and studies have shown the DFT not to be a predictor of long-term survival. Furthermore, there are risks in DFT measurement. If in atrial fibrillation, defibrillation might restore sinus rhythm and lead to left atrial thrombus dislodgement. Occasionally, prolonged resuscitation may be needed and very occasionally it might not be successful. The argument against routine DFT testing is greater in patients receiving a device for primary prevention (see below), in that risks have to be weighed against the possibility that ventricular fibrillation might never spontaneously occur. Many centres do not now routinely measure the DFT. Testing should, however, be carried out in patients with hypertrophic cardiomyopathy, where high DFTs are often encountered.
Subcutaneous ICD
Recently, an entirely subcutaneous ICD system has been introduced which obviates the challenges of venous access and complications associated with transvenous leads. It consists of a lead that has a shocking coil and sensing electrodes positioned subcutaneously parallel to the left edge of the sternum together with a pulse generator implanted subcutaneously in the region of the left anterior axillary line. It is suitable for patients at risk of sudden death who do not have indications for cardiac pacing.
Indications for ICD implanation
Indications are considered in terms of primary and secondary prevention. Primary prevention is therapy for patients who are at high risk of death from ventricular tachycardia or fibrillation who have not yet sustained these arrhythmias. Secondary prevention is therapy to deal with patients who have experienced ventricular tachycardia or fibrillation.
UK guidelines (National Institute for Health and Clinical Excellence)
Secondary prevention
Several major clinical trials have demonstrated that in the above groups of patients, an ICD reduces mortality as compared with antiarrhythmic drug therapy. Overall, these studies have shown a reduction in death due to cardiac causes of approximately 50% and a reduction in ‘all-cause mortality’ of approximately 24%. A survey has shown that over a three-year period one life is saved for every 4–5 defibrillators implanted.
Primary prevention
These recommendations are based on studies that have shown that mortality in patients with the above characteristics is reduced by an ICD as compared with antiarrhythmic drug therapy, mainly amiodarone.
The main indicators of high risk in conditions (i)–(iv) are discussed in chapters 12 and 13. Following repair of Fallot’s tetralogy, prolonged QRS duration and ventricular dysfunction have been reported to be predictors of sudden death.
Further indications for primary prevention relevant to UK guidelines
Three recent studies have shown that in patients with poor left ventricular function (ejection fraction < 35%), whether due to coronary artery disease or to cardiomyopathy, and New York Heart Association class II or III heart failure, prognosis can be improved by ICD implantation even without there being non-sustained ventricular tachycardia or a positive ventricular stimulation study.
Analysis of these studies has shown that a policy of ICD implantation in patients with poor ventricular function will prolong life, on average, by 2–6 years and is ‘cost-effective’. These studies have also clearly shown that antiarrhythmic therapy, mainly amiodarone, though it may reduce the incidence of ventricular arrhythmia, does not improve prognosis.
Recent myocardial infarction
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree