Although pulse generator implantation is usually straightforward and free of adverse events, there are multiple potential complications that can occur. Prior to device implant, not only should the procedure be explained in detail to the patient, but potential complications should be discussed and that discussion documented in the permanent medical record. Our practice is to routinely discuss the complications that are the most common or that carry the greatest threat to the patient. This includes lead dislodgement, pocket bleeding, pneumothorax, infection, and cardiac perforation with tamponade. With implantable cardioverter-defibrillator (ICD) implantation we also discuss complications associated with defibrillation threshold (DFT) testing, as well as the potential need for additional hardware in order to achieve an adequate DFT. If a cardiac resynchronization therapy (CRT) system is being implanted, additional time should be spent discussing problems that can arise with placement of the coronary sinus (CS) lead.
Complications Related Directly to the Implant Procedure
Complications of lead placement result from cannulation of the vein, catheterization of the heart, and from placement of a permanent lead.
Lead Dislodgement
Historically, the most common complication of transvenous pacing has been lead dislodgement. Improved fixation mechanisms have substantially reduced the frequency of this complication for both atrial and ventricular pacing leads. Secondary intervention rates for all reasons should be <2% for ventricular leads and <3% for atrial leads. Some experienced implanters would argue that dislodgement rates should be even lower, e.g., 1% for right ventricular (RV) leads and 2% for atrial leads. In the Pacemaker Selection in the Elderly (PASE) trial, lead dislodgement was the most common complication, occurring in 9 of the 407 patients, or 2.2%.1 In the REPLACE registry of patients undergoing pacemaker or ICD generator replacements, lead dislodgement was the most common major complication with a 6-month incidence of 1.0% in patients undergoing generator replacement alone vs. 7.9% in patients undergoing generator replacement and lead implantation (the majority of patients had an upgrade to a CRT device).2 Procedures involving placement of additional leads (upgrade) may be complex as a result of obstructed veins or thrombosis, and extensive manipulation may result in either dislodgement of the previously existing lead or lead damage. These data suggests that lead dislodgement rates for CS leads may be even higher. Slightly higher dislodgement rates may also be tolerable in pediatric patients, whose activity is more difficult to control, and in patients with unusual anatomy, such as congenital cardiac anomalies.
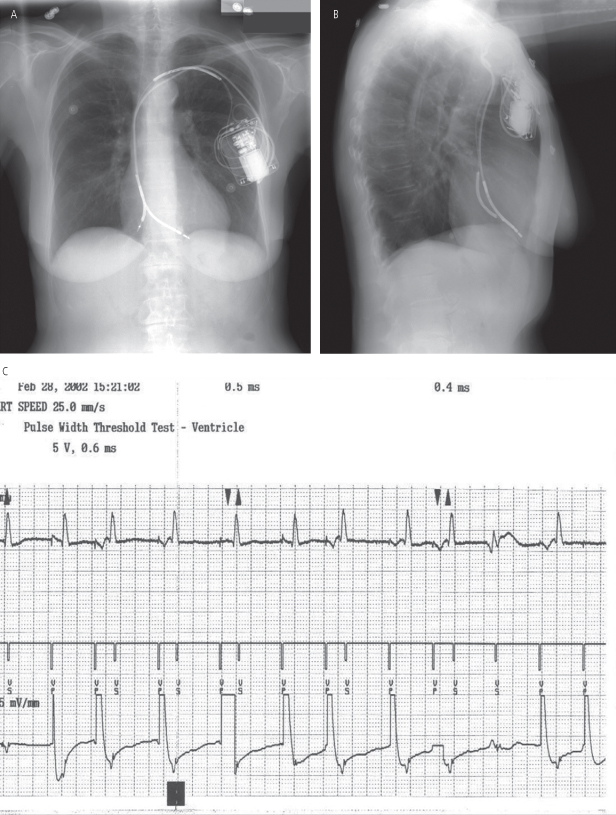
Dislodgement is often classified as “macrodislodgement” or “microdislodgement.” Macrodislodgement is radiographically evident, but microdislodgement is not (Fig. 6.1). Adequate lead position is assessed by posteroanterior and lateral chest radiographs (see Chapter 11: Radiography of Implantable Devices). Lead placement by chest radiography may appear excellent in the patient with microdislodgement, but the tip has moved sufficiently to impair myocardial contact and function. Due to beat-to-beat variability in cardiac and lead position, only gross lead movement is identified as macrodislodgement.
Fig. 6.1 Posteroanterior (A) and lateral (B) chest radiographs on the day after implantable cardioverter-defibrillator (ICD) implantation. The fluoroscopic image at the end of the implant had demonstrated a right ventricular apical position and adequate “J” on the atrial lead. However, on the X-ray shown, both atrial and ventricular lead positioning is suboptimal due to gross dislodgment. It appears that neither was adequately secured and have pulled back to a more shallow position. (C) The tracing was obtained when the ICD was programmed to the VVI pacing mode. Note complete failure to capture.
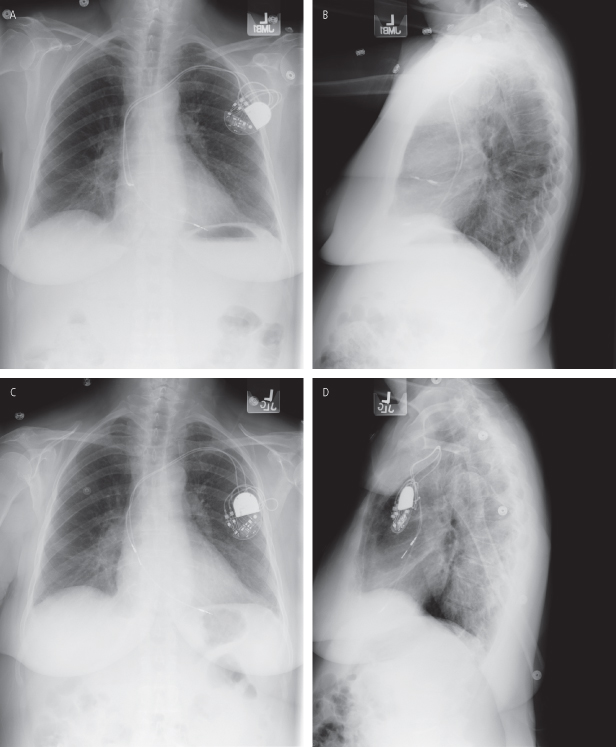
The atrial lead dislodgement rate has traditionally been higher than the ventricular dislodgement rate (Fig. 6.2). The optimal atrial lead design remains uncertain. A retrospective study found the lowest rate of lead dislodgement with straight “J-post shaped” active fixation atrial leads compared with active and passive fixation J-shaped leads.3 A prospective study, contrastingly, found that straight leads, as compared with preformed J leads, are more likely to dislodge but less likely to malfunction.4 In another prospective comparison of atrial passive and active fixation J-shaped leads, both performed well. Passive fixation leads, however, required less fluoroscopy at implantation, had better thresholds, and had a considerably lower rate of pericardial irritation or effusion.5 As newer, smaller leads become available, performance characteristics will further evolve. Atrial lead dislodgement may be related more to implanter experience than the fixation mechanism or shape. The geometry and pathology of the atrium may also dictate atrial lead type. In general, preformed atrial leads may be difficult to use when a large atrium or surgical scarring requires shaping the lead (stylet) to access a particular location. In unusual circumstances, a lead delivery system similar to those used for cardiac resynchronization may be necessary to place a nonpreformed lead.
Fig. 6.2 (A) Posteroanterior (PA) and (B) lateral radiographs on the morning after implantation of a dual-chamber pacemaker. The active-fixation atrial lead appears to be somewhat shallow on the PA film but reasonable “J” configuration of the lead is noted on the lateral film. Atrial thresholds were excellent. (C) PA and (D) lateral radiographs obtained a few days later when the patient presented with vague fatigue. The atrial lead has clearly dislodged, being most evident on the lateral view.
The dislodgement rate of CS leads tends to be higher for several reasons. In addition to microdislodgement and macrodislodgement giving rise to inadequate thresholds being made more likely with the complex venous system, even with adequate thresholds, phrenic nerve stimulation or inadequate resynchronization may occur as a result of lead dislodgement. A novel active fixation CS lead has a low dislodgement rate, although it may potentially be more difficult to extract.6 Patients scheduled to receive a CS lead should be counseled regarding the importance of checking for adequate resynchronization (EKG, echo) in addition to the need for DFT testing, if applicable. Furthermore, they should be informed of a higher necessity for lead revision.
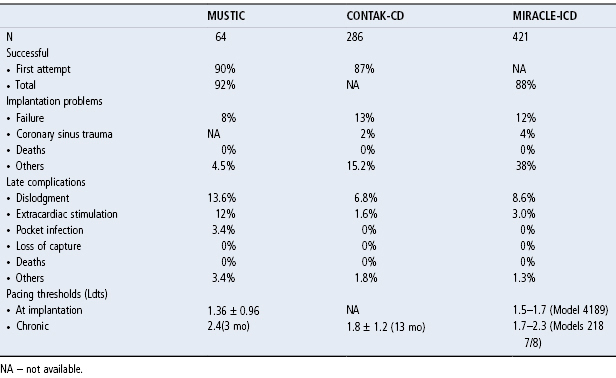
Complications of CS lead placement, including dislodgement, from MUSTIC, CONTAK-CD, and MIRACLE-ICD trials, have been summarized in an Expert Consensus Statement: Resynchronization Therapy for Heart Failure from the Heart Rhythm Society.7 A table from that consensus statement summarizing CS lead implantation success rates, implant problems, complications, and thresholds is shown in Table 6.1.
Table 6.1 Complications of coronary sinus lead placement.
(Reproduced with permission from Saxon LA, De Marco T, Prystowsky EN, et al. www.hrsonline.org/ClinicalGuidance/upload/resynch_therapy_HF.pdf. [accessed 14 September 2012]
Pneumothorax
Complications of venous entry, inherent in any approach to venous structures, include damage to associated arterial or neural structures, extensive bleeding, air embolism, and thrombosis. With the subclavian approach, the potential for pneumothorax also exists. This can be minimized by knowledge of the patient’s anatomy, attention to detail, and contrast venography (Fig. 6.3). In an older multicenter trial, pneumothorax occurred in 2.0% of patients and was more common in older patients and patients with lower body mass indices (<20 kg/m2).1 A recent systematic review reported a 0.9% incidence of pneumothorax with either ICD or CRT implantation.8
Fig. 6.3 Posteroanterior radiograph taken within a few hours after implantation in a patient complaining of mild dyspnea and left-sided chest discomfort. The patient has a substantial pneumothorax on the left as a complication of subclavian puncture.
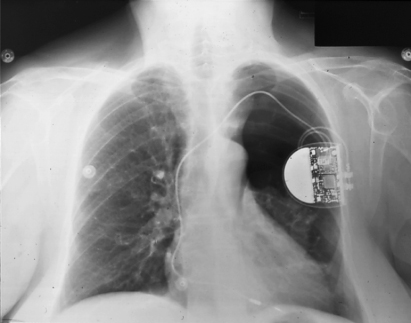
It has been reported that when experienced implanters use the subclavian puncture technique, the incidence of pneumothorax approaches 1%.9 As noted in Chapter 5: Implantation techniques, peripheral injection of contrast media and fluoroscopic guidance of the subclavian puncture may help to minimize complications (Figs 5.6 and 5.7).10 In addition, the regional anatomy must be considered before subclavian puncture is undertaken. In a patient with unusual anatomy of the chest wall or clavicle, the subclavian vein can be displaced, and the usual subclavian puncture landmarks can be altered. Care must also be taken in the kyphotic patient in whom the venous anatomy may be displaced. Subclavian arterial dilatation or aneurysm may displace the targeted veins. Direct ultrasound visualization using a handheld device can help avoid contrast and yet identify the vein before it enters the thorax. However, caution is necessary to avoid contamination of the field, and placing the needle into the vein while continuing to visualize the vessel after a relatively small pocket has been created can be challenging. Excessive contrast use (obviated with this technique) has been linked with poorer outcomes including transient renal insufficiency.11
If a pneumothorax develops, it may manifest during the pacemaker procedure or as late as 48 h after implantation. Indications of pneumothorax are aspiration of air during subclavian puncture when the exploring needle is either introduced or removed, unexplained hypotension, chest pain, or respiratory distress.
If the subclavian artery is lacerated, hemopneumothorax may occur (Fig. 6.4), or bleeding may occur into the tissues, resulting in hematoma formation (Fig. 6.5). Other potential complications of subclavian venous entry are air embolism, arteriovenous fistula, thoracic duct injury, and brachial plexus injury. Although all are uncommon, it is essential that the implanter using the subclavian puncture technique be familiar with the potential problems.
Fig. 6.4 Posteroanterior chest radiograph from a patient with an internal cardioverter-defibrillator implanted on the right side. There are multiple complications that are evident radiographically. The patient initially had a device on the left side, which was removed, but there is a retained sponge in the abandoned pocket. Close inspection at the left upper cardiac border reveals air in the pericardial space. The arrow points to a faint line which represents the pericardium that is separated from the cardiac surface by a pneumopericardium. On the right side of the chest the upper arrow points to a pneumothorax. The lower arrow on the right side of the chest points to a fluid level that represents an accumulation in the chest, most likely a hemothorax.
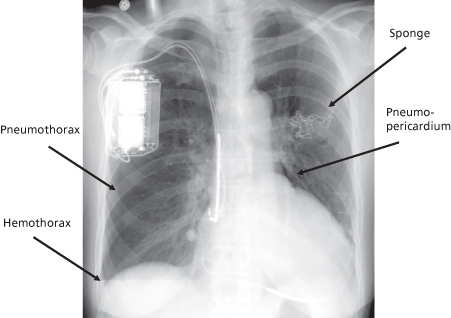
Fig. 6.5 Posteroanterior chest radiograph of a patient following attempted pacemaker implantation. During the procedure the subclavian artery was inadvertently punctured. On the radiograph shown, there is fullness of the axillary and left lateral chest and a density can be appreciated above the left breast. This represents hematoma formation which corresponded to a substantial fall in the patient’s hemoglobin.
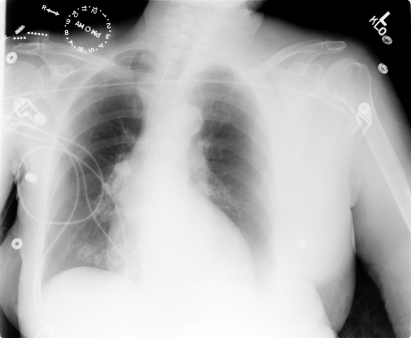
After puncture of the subclavian or axillary vein, a chest radiograph should be obtained and inspected specifically for pneumothorax. A pneumothorax estimated to involve <10% of the pleural space can probably be observed without chest tube placement. A chest tube should be considered if >10% of the lung is involved, the patient has continued respiratory distress, or hemopneumothorax is present.
Lead Perforation
Cardiac perforation during lead implantation is among the most potentially serious complications that occur with device procedures. Anatomically, the free wall of the right atrium and right ventricle as well as the distal coronary venous system is extremely thin and prone to perforation. CT scan studies have revealed a high rate of asymptomatic and otherwise clinically unrecognized perforations. Care with avoiding the free wall and placing leads whenever possible onto the septum decreases the chance of significant perforation.
The true frequency of lead perforation is difficult to determine and varies widely depending on the series and types of leads evaluated. Perforation has been shown to occur in 0.1–0.8% of patients undergoing pacemaker implantation and 0.6–5.2% in patients undergoing ICD implantation.2,10,12 The incidence of coronary vein dissection and coronary vein perforation with CS lead placement has each been reported at 1.3%.8
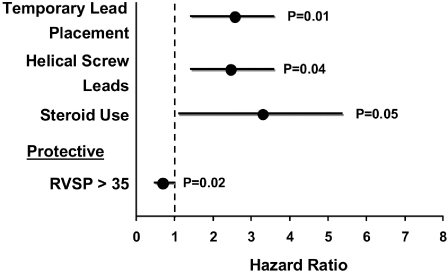
Perforations are more common in elderly patients in whom the RV wall may be thinner. Anecdotally, the risk is higher in elderly women than men. In patients in whom a post-implant pericardial effusion was present and was believed to be a consequence of perforation, risk factors included the presence of a temporary pacemaker, helical screw leads, and systemic steroid use (Fig. 6.6). The only protective factor was RV systolic pressure >35 mmHg.12
Fig. 6.6 Multivariate hazard ratios for development of pericardial effusion and symptoms consistent with cardiac perforation following permanent pacemaker placement. RVSP, right ventricular systolic pressure.
(Reprinted from Mahapatra S, Bybee KA, Bunch TJ, et al. Incidence and predictors of cardiac perforation after permanent pacemaker placement. Heart Rhythm 2005; 2:907–11, with permission.)
Diagnosis of a perforation is usually based on clinical findings. Echocardiography may suggest perforation, but unless the lead is completely through the myocardium, the study may be inconclusive. More recently, computed tomography (CT) has been reported as a method of diagnosing myocardial perforation (Fig. 6.7).13 In a retrospective review of CT scans obtained in 100 patients with prior device implantation, there was a surprisingly high incidence of radiographically evident perforations. Overall, 15% of patients had a myocardial perforation. Rates of perforation were 15% for atrial leads and 6% for ventricular leads. There was perforation of 14% of RV ICD leads and 3% of RV pacemaker leads. Active fixation right atrial leads had a 12% perforation rate, and passive fixation atrial leads had a 25% perforation rate. Of RV pacemaker leads, 7% of active fixation and 5% of passive fixation leads demonstrated perforation. When correlated with measured pacing values, there was no difference in impedance between perforated and nonperforated leads. Only one perforated ventricular lead was said to have a “high” threshold.14 In an older autopsy study, lead perforation was seen in 27% of patients with right atrial leads.15 The high incidence of asymptomatic radiographic and autopsy identified myocardial perforations suggests that they are not uniformly detrimental.
Fig. 6.7 Axial image from a 64-detector ECG gated computed tomography with IV contrast material in a 73-year-old man. There is a circumscribed mass adjacent to the right atrial appendage presumed to be a hematoma (arrow). A portion of the pacemaker lead (arrowhead) is seen outside of the right atrial appendage in the presumed mediastinal hematoma.
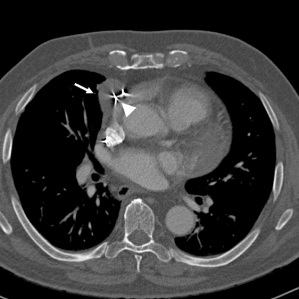
Perforation can present in multiple ways. It may potentially be asymptomatic and detected radiographically or by a rising stimulation threshold. In other patients, signs may include right bundle branch block (RBBB)-paced rhythm in a patient in whom the lead is placed in the RV (Fig. 6.8), intercostal muscle, or diaphragmatic stimulation, friction rub after implantation, pericarditis, pericardial effusion, and cardiac tamponade. (Note: When the lead is placed in the RV, because left ventricular (LV) activation is late, a left bundle branch block (LBBB) pattern is expected. However, even in the absence of cardiac perforation and LV stimulation which routinely results in RBBB pattern when the RV is abnormal or the heart is rotated, apical RV pacing may result in RBBB pattern during stimulation even in the absence of perforation.) Hemodynamic deterioration may occur at the time of perforation, but a “slow” pericardial leak may also arise, and symptoms may not appear for 24–48 h. Delayed perforation, i.e., >1 month, although rare, has also been described.16
Fig. 6.8 Twelve-lead electrocardiogram obtained immediately after VVI pacemaker implantation. The paced ventricular complex has a right bundle branch block configuration compatible with left ventricular lead placement.
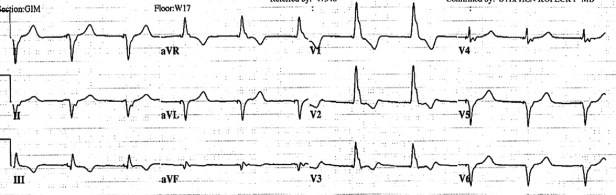
If the patient has mild symptoms or signs compatible with lead perforation, such as pericardial pain and friction rub, but a persistent perforation cannot be identified, observation is reasonable. If the symptoms or signs resolve within 24–48 hours, lead repositioning may not be necessary. If an echocardiogram reveals a small pericardial effusion but no definite perforation, serial echocardiograms should be obtained to be certain that the effusion is not hemodynamically significant or enlarging.
Management of lead perforation depends, in part, on the clinical sequelae. Perforation associated with hemodynamic compromise must be dealt with as an emergency. If clinical and echocardiographic findings are consistent with tamponade, echocardiographically guided pericardiocentesis should be performed. Usually, placing an indwelling pigtail catheter is reasonable to avoid recurrent hemodynamic compromise and to measure drainage accurately. If neither significant additional drainage nor reaccumulation occurs by echocardiography, the catheter can be removed in 48–72 hours and the patient managed by observation and reimaging. If no reaccumulation occurs, the leads may not have to be repositioned so long as thresholds remain stable. With acute perforation, any significant rise in threshold necessitates lead withdrawal and repositioning. With delayed perforation and a rising lead threshold, depending on the lead position, one might consider a conservative approach with placement of a new lead rather than withdrawal or repositioning of the perforated lead.17 Any time a lead suspected of perforation is withdrawn, there is the potential for pericardial bleeding. In our institution, we do this in our usual pacemaker implant suite with the echocardiographer and necessary equipment for echocardiographic-guided pericardiocentesis standing by. Others prefer to retract the lead suspected of perforation in an operating room with a cardiac surgeon on standby.18
Late complications of lead perforation may occur if lead extraction is required. As discussed above, lead perforation is probably more common than realized, and patients may do well over the long term unless lead extraction is required. If there has been transmyocardial perforation or perforation and/or dissection of the venous system with re-entrance into the circulation at the time of implant, catastrophic results may occur when trying to extract these leads either with traction or a laser extraction system. Careful review of the patient’s implantation and associated images (however remote) is recommended when questions arise prior to performing lead extraction.
Perforation of the great vessels may also occur as a procedural complication. Management would be dependent on the patient’s symptoms and hemodynamic stability (Fig. 6.9).
Fig. 6.9 Still-frame fluoroscopic image obtained at the time of upgrade from a dual-chamber pacemaker to a CRT-D system. As the new ventricular lead was passed through the venous system, the lead perforated, probably at the level of the superior vena cava. Because of uncertainty of the lead position, a contrast injection was performed and contrast is apparent in the mediastinum. The patient remained asymptomatic and hemodynamically stable. The lead was withdrawn and the procedure successfully completed.
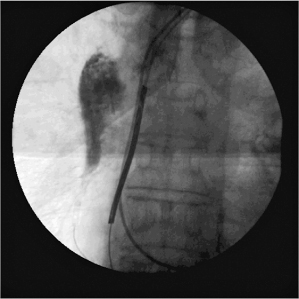
Pericarditis
Clinical findings of pericarditis, as mentioned, may be associated with lead perforation. However, pericarditis may occur with or without any other clinical evidence of perforation. It is possible for the tip of an active fixation lead to irritate the pericardium, most commonly a right atrial active fixation lead.19,20 If there is no evidence of tamponade or symptomatic pericardial effusion, it is reasonable initially to treat the patient conservatively, i.e., observation and pain medications. Anti-inflammatory medications, e.g., nonsteroidal anti-inflammatory drugs or steroids, may relieve symptoms. However, if the medications cannot be withdrawn without symptom recurrence, it may be necessary to remove and reposition the lead.
Arrhythmias
A frequent complication during lead implantation is development of supraventricular or ventricular arrhythmias related to lead manipulation. These effects are usually transient, ending promptly when the lead position is changed. Rarely, they may be sustained. Atrial manipulation may rarely result in sustained atrial tachycardia, fibrillation, or flutter, which complicates placement of a permanent atrial lead. Atrial tachycardia or flutter may revert to normal sinus rhythm with gentle manipulation of the electrode against the atrial wall or by overdrive pacing. Commonly used pacing system analyzers have a “temporary” overdrive-pacing mode available that allows rapid pacing. If the patient is in atrial tachycardia or flutter, burst overdrive pacing via the pacing system analyzer may interrupt the tachyarrhythmia and restore normal sinus rhythm.
Management of atrial fibrillation is more difficult and may require cardioversion to restore normal sinus rhythm during the implant procedure. Prior to cardioversion, the patient’s arrhythmia history and anticoagulation history should be reviewed to be certain cardioversion is safe. We routinely place transcutaneous “pacing pads” which can be used for cardioversion in the event that cardioversion is necessary. Moreover, we routinely perform device implantation in anticoagulated patients, as long as the international normalized ratio (INR) is <2.5. Patients who are not anticoagulated and who have been in atrial fibrillation or flutter for >48 hours are generally not cardioverted because of the potential risk of thromboembolism and stroke.21 Brief ventricular arrhythmias are also common, particularly during ventricular lead manipulation. They are usually easily controlled. However, in patients with a history of spontaneous sustained ventricular tachycardia, manipulation of the lead may initiate ventricular tachyarrhythmias. Occurrence is obviously more likely during implantation of an ICD. For this reason, all pacemaker and ICD recipients are monitored, and life-support equipment and an external defibrillator are immediately available.
Ventricular extrasystoles may occur in the early post-implantation period as a result of irritation at the electrode–myocardium interface. These premature beats, termed “tip extrasystoles,” are usually of the same morphology as the paced ventricular beat (Fig. 6.10). They usually subside within 24 hours after implantation and rarely, if ever, require treatment. In patients receiving a defibrillator with frequent lead-related ectopy or nonsustained VT seen at the end of the procedure or during post-procedure observation, device detections and therapies may be programmed off temporarily and the patient observed to see that lead-related ectopy and arrhythmia have subsided. If they do not subside in a day, consideration for moving the lead to an alternate location should be given.
Fig. 6.10 Electrocardiographic tracing obtained within hours after VVI pacemaker implantation. The pacemaker is programmed to a lower rate of 50 ppm. There are frequent ventricular extrasystoles morphologically similar to the paced beats, and at least one of the premature beats is undersensed.
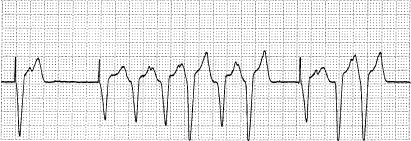
In addition to tachyarrhythmias, bradyarrhythmias may occur during implant. In patients with intermittent atrioventricular (AV) block and LBBB, catheter trauma to the right bundle may result in AV block. More commonly, bradycardia follows overdrive suppression of an escape ventricular focus during threshold testing. In a patient at high risk for development of asystole or complete heart block during the procedure, a temporary pacemaker may be placed before implantation. Alternatively, external pacing pads can be placed during the procedure should temporary pacing be needed. This method may obviate adjunctive transvenous temporary pacing. At our institution we routinely place external pacing pads.
Pulse Generator Pocket Complications
Pocket hematomas are a frequent complication of permanent pacemaker and ICD implantation, occurring in 4.9–9.5% of patients.22–24 Bleeding complications are more likely to occur in patients on antiplatelet agents or anticoagulants. While aspirin alone does not appear to increase the risk for pocket hematomas, the combination of aspirin and clopidogrel increases the incidence of hematomas to 7.2–24.2%.22,23 Renal dysfunction also increases the risk for periprocedureal bleeding.25 Patients should be informed of the risk for bleeding complications related to device implantation.
The provider must consider the indications for antiplatelet agents and anticoagulants prior to device implantation. It is not our routine practice to discontinue antiplatelet agents. In patients at high risk for thromboembolism, continuing oral anticoagulation with warfarin is preferred to bridging with low-molecular-weight and unfractionated heparin.26 A retrospective study found a substantially higher risk for hematoma with no difference in thromboembolism in patients on bridging anticoagulation vs. continued warfarin (mean INR 2.57).27,28 A prospective study in patients undergoing implantation of CRT devices found a four times higher risk for pocket hematoma in patients on bridging anticoagulation compared with continued warfarin (mean INR 2.39).29 In patients requiring oral anticoagulants (warfarin), we like the INR to be <2.5 at the time of implantation. For many patients, this may not require any alteration in therapy. If the patient is maintained at a higher INR, holding the warfarin for one to two nights prior to implantation will generally result in the INR being at acceptable levels to proceed. As noted previously in this chapter, unfractionated heparin or low-molecular-weight heparin are always discontinued prior to device implant and ideally avoided for a minimum of 24 hours post-implantation. Periprocedural management of newer oral anticoagulants, such as dabigatran, remains uncertain.
Careful local hemostasis during implantation is essential. Special steps can be considered at the time of implant in patients with excessive “oozing” within the pocket. Materials that can be used in patients with excessive oozing refractory to electrocautery include Gelfoam, thrombin-treated biodegradable mesh, and topical application of thrombin, which can be highly effective in stopping the bleeding. If topical thrombin is used, it may interfere with subsequent INR measurements.
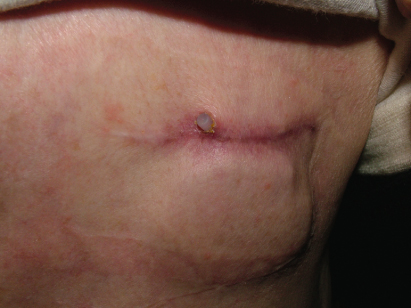
Because local ecchymoses are common after pacemaker implantation, an ecchymosis, regardless of size, that is not expanding is treated by observation only. Discrete hematoma formation at the site must be dealt with on the basis of its secondary consequences (Figs 6.11 and 6.12). Should a substantial hematoma occur, conservative treatment is preferred, if possible. If bleeding continues, pain cannot be managed with mild analgesics, or the integrity of the incision is threatened, evacuating the hematoma should be considered. Aspiration of the hematoma or placement of a drain should not be attempted, because it is often ineffective, and regardless of the care taken to maintain sterile technique, increases the risk of infection. If hematoma evacuation is required, the procedure should be thoroughly sterile.
Fig. 6.11 Extensive ecchymoses following device implantation. The patient was on aspirin therapy at the time of the procedure. A portion of the left arm ecchymoses was secondary to an earlier unrelated procedure. The ecchymoses resolved without any adverse effects.
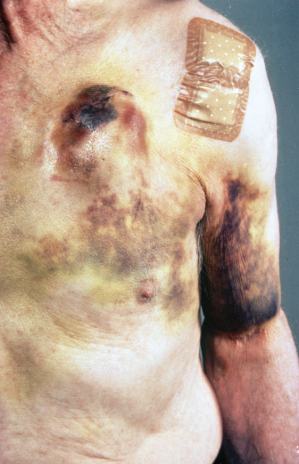
Fig. 6.12 (A) Front and (B) side photos of a patient after device implantation. The patient has a significant hematoma. Discomfort was easily managed with analgesics and there was no threat to the integrity of the incision. The hematoma gradually resolved without consequence.
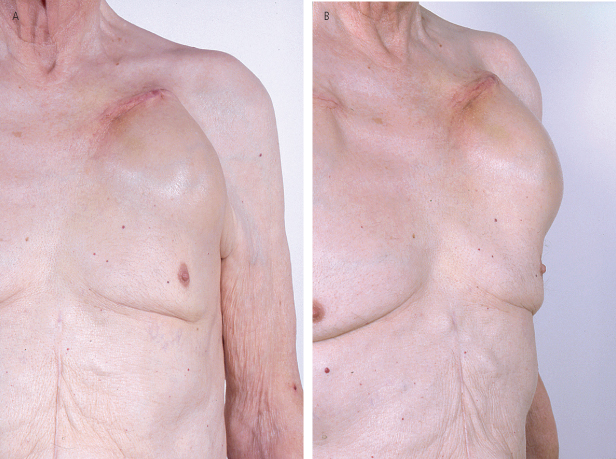
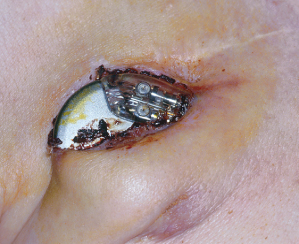
Late complications, including erosion and migration, are often the result of suboptimal initial surgery or infection (Figs 6.13 and 6.14). These can be minimized by careful technique at the time of initial pacemaker implantation and by the formation of an adequate pocket. A painful pocket may also result from inadequate positioning of the pacemaker below the subcutaneous tissues, and the pulse generator may have to be repositioned.
Fig. 6.13 Erosion of a pacemaker. The patient denied any discomfort and stated that he had been able to see some portion of the device for at least 3 months prior to seeking medical attention.
Pain
Patients should be told to expect some local discomfort at the pacemaker implantation site. This gradually subsides and can usually be managed with mild analgesics, such as acetaminophen. A painful pacemaker site, commonly called a “painful pocket,” can occur for several reasons and should be taken seriously. The differential diagnosis includes:
- Infection
- Pacemaker implanted too superficially
- Pacemaker implanted too laterally
- Pacemaker allergy.
An indolent infection may manifest as a painful pocket long before any other signs of infection. This diagnosis can be difficult. Needle aspiration of a pacemaker site is not advised for fear of introducing infection. However, if a painful pocket is explored for any reason, culture specimens should be obtained.
The pacemaker pocket should be formed in the prepectoralis fascia, i.e., deep to adipose tissue in the subcutaneous space. If it is placed anterior to the adipose layer, i.e., within subcutaneous tissues, significant pain may result. This is one of the most common causes of a painful pocket and justifies revision of the pacemaker pocket.
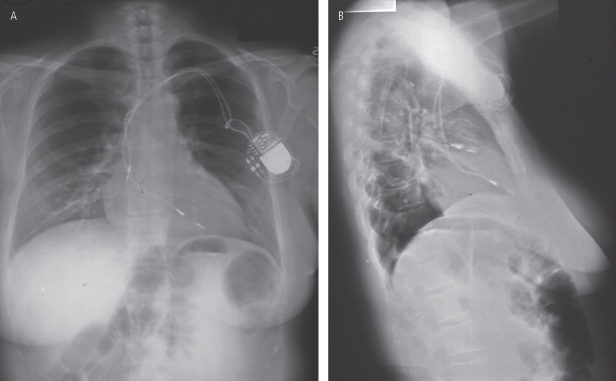
If the pacemaker is positioned too laterally, impingement on the axillary space may cause discomfort (Fig. 6.15). Although there are published series on axillary pocket placement, substantial experience is required to position the pacemaker in such a way that there is no discomfort.30
Fig. 6.15 (A) Posteroanterior and (B) lateral radiograph from a patient with chronic pain and arm limitation after pacemaker implantation. An attempt had been made to place the pacemaker in an axillary position for cosmetic reasons. From this single view it also appears that both leads are “shallow,” i.e., suboptimal redundancy on the ventricular lead and suboptimal “J” on the atrial lead.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree