Marathon running commonly causes a transient elevation of creatine kinase and cardiac troponin I (cTnI). The use of statins before marathon running exacerbates the release of creatine kinase from skeletal muscle, but the effect of statin use on exercise-induced cTnI release is unknown. We therefore measured cTnI concentrations in statin-using (n = 30) and nonstatin-using (n = 41) runners who participated in the 2011 Boston Marathon. All runners provided venous blood samples the day before, within an hour of finishing, and 24 hours after the marathon. cTnI was assessed at each time point via both a contemporary cTnI and high-sensitivity cTnI (hsTnI) assay. Before the marathon, cTnI was detectable in 99% of runners with the use of the hsTnI assay. All participants completed the marathon (finish time: 4:04:09 ± 0:41:10), and none had symptoms of an acute coronary syndrome. cTnI increased in all runners (p <0.001) immediately after the marathon, and half (hsTnI = 54% vs contemporary cTnI = 47%) exceeded the diagnostic cut-point for an acute myocardial infarction. Statin use did not affect the magnitude of cTnI release (group*time p = 0.47) or the incidence of runners with cTnI elevation greater than the diagnostic cut-point for myocardial infarction (57% vs 51%, p = 0.65). In addition, there was no significant association between statin potency and cTnI release (r = 0.09, p = 0.65). In conclusion, marathon-induced cTnI increases are not altered by statin use.
Cardiac troponin I (cTnI) is a sensitive and specific marker of myocardial injury, but serum cTnI increases may occur after strenuous physical exercise in healthy individuals. Marathon participants often demonstrate cTnI elevations after racing that are similar to those characteristic of an acute coronary syndrome. The mechanism mediating exercise-induced cTnI release remains uncertain, and it is unclear why some runners demonstrate increased cTnI concentrations and others do not. Skeletal muscle injury, documented by an increase in serum creatine kinase (CK) concentrations, commonly occurs after a marathon run. The use of 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitors (i.e., statins) before a marathon increases the magnitude of CK release from skeletal muscle, but the effect of statin use on myocardial cTnI release after prolonged aerobic exercise has not to our knowledge been examined. We measured serial cTnI concentrations by using both a contemporary cTnI and high-sensitivity cTnI assay (hsTnI) in statin-using and nonstatin-using runners who participated in the 2011 Boston Marathon. We hypothesized that statin-using runners would demonstrate greater cTnI elevations than otherwise-matched, nonstatin-using runners.
Methods
We recruited 30 statin-using runners (statin group) and 41 nonstatin-using runners (control group) participating in the 2011 Boston Marathon. All participants provided written, informed consent (Institutional Review Board, Hartford Hospital, Hartford, Connecticut). Participants were nonsmokers, aged >35 years, and free of known cardiovascular or metabolic disease except for dyslipidemia for the statin group.
The day before the marathon (PRE), participants provided their medical history and training mileage for the 3 months and 1 week preceding the marathon. Resting blood pressure, heart rate (Welch Allen 52000 Vital Signs Monitor, Skaneateles Falls, New York), height, and body mass were measured. Venous blood samples (25 ml) were obtained before (PRE), within 1 hour after (FINISH), and ∼24 hours after the race (POST-24H).
Venous blood was collected in serum-gel Vacutainer tubes (BD, Franklin Lakes, New Jersey) and allowed to clot for ∼45 minutes. Serum was separated by centrifugation, aliquoted, frozen, and stored at −80°C for later analysis. Cholesterol and triglycerides, hemoglobin, hematocrit, albumin, bilirubin (total and direct), alkaline phosphatase, aspartate aminotransferase, and alanine aminotransferase were determined after a 12-hour fast at PRE only (Quest Diagnostics, Nichols Institute, Chantilly, Virginia). Lipid and hepatic panels were performed with a spectrophotometric assay. The hematocrit and hemoglobin were measured with a colorimetric assay. Muscle myoglobin was assessed with a nephelometric assay.
cTnI was analyzed with a contemporary assay (Siemens Dimension Vista cTnI; Siemens Healthcare Diagnostics Inc., Newark, New Jersey) and a precommercial, high-sensitivity assay (i.e., hsTnI; Siemens Vista hsTnI, Siemens Healthcare Diagnostics Inc.). The limit of detection (LOD) of the contemporary cTnI assay was 15 ng/L, with a 10% coefficient of variation (CV) at 40 ng/L. A concentration of 45 ng/L (10% CV) has been reported as the 99th percentile upper reference limit for the contemporary cTnI assay and has been proposed as the clinical cut-point for the diagnosis of acute myocardial infarction. The LOD of the hsTnI assay was 0.8 ng/L, with an 8.5% CV at 4.4 ng/L and a 99th percentile of 48 ng/L (5.0% CV). All samples were analyzed in duplicate and mean values calculated. We also measured complementary cardiovascular biomarkers (Siemens Dimension Vista Intelligen Lab System, Siemens Healthcare Diagnostics Inc.): N-terminal pro–B-type natriuretic peptide (NT-proBNP), myoglobin, and cystatin C at PRE, FINISH, and POST-24H.
All data were reported as mean ± SD unless stated otherwise, and statistical significance was set at a p-value <0.05. Statistical analyses were performed with the Statistical Package for the Social Sciences (IBM SPSS Statistics for Windows, Version 20.0, IBM Corp., Armonk, New York). The normality of the data was examined by the Kolmogorov-Smirnov test. When data demonstrated a non-Gaussian distribution, natural logarithmic transformation was applied. Changes in contemporary cTnI and hsTnI concentrations over time were assessed with a repeated measurements analysis of variance, with post-hoc Bonferroni corrections. The difference in the number of participants greater than the LOD and the 99th percentile with the contemporary cTnI and hsTnI assays was tested for significance by chi-square analysis. To determine the cardiac specificity of the contemporary cTnI and hsTnI assays, FINISH TnI concentrations were correlated with a Spearman correlation coefficient. Differences between the statin and control group were assessed with a Student t test and chi-square test for continuous and nominal parameters, respectively. To investigate the effect of statin dose on contemporary cTnI and hsTnI release (Spearman correlations), the statins were classified by the expected potency of cholesterol reduction according to dose equivalencies: rosuvastatin 2.5 mg = atorvastatin 5 mg = simvastatin 10 mg = lovastatin 20 mg = pravastatin 20 mg = fluvastatin 40 mg.
Results
All athletes ( Tables 1, 2 ) completed the marathon with an average race time of 4:04:09 ± 0:41:10 (range, 2:48:58–5:46:41 minutes). No runners had symptoms suggestive of an acute coronary syndrome within 24 hours of race completion.
| Groups | Total (n = 71) | Statin (n = 30) | Control (n = 41) | Statin vs. Control (p-Value) |
|---|---|---|---|---|
| Men:women (number) | 51:20 | 23:7 | 28:13 | 0.44 |
| Age (years) | 53 ± 8 | 56 ± 8 | 51 ± 7 | 0.003 |
| Height (meters) | 1.73 ± 0.10 | 1.74 ± 0.09 | 1.73 ± 0.11 | 0.70 |
| Weight (kg) | 70.0 ± 12.2 | 71.1 ± 11.1 | 69.4 ± 13.0 | 0.56 |
| Body mass index (kg/m 2 ) | 23.1 ± 2.7 | 23.3 ± 2.5 | 23.1 ± 2.9 | 0.71 |
| Resting heart rate (bpm) | 59 ± 11 | 59 ± 13 | 59 ± 10 | 0.99 |
| Systolic blood pressure (mmHg) | 138 ± 18 | 141 ± 17 | 137 ± 17 | 0.41 |
| Diastolic blood pressure (mmHg) | 79 ± 11 | 78 ± 16 | 79 ± 11 | 0.77 |
| Average running distance (miles/week) | 38 ± 16 | 39 ± 19 | 39 ± 13 | 0.85 |
| Running distance week premarathon (miles) | 20 ± 13 | 22 ± 17 | 19 ± 10 | 0.29 |
| Total cholesterol (mg/dl) | 183 ± 35 | 169 ± 32 | 193 ± 32 | 0.003 |
| High-density lipoprotein cholesterol (mg/dl) | 71 ± 19 | 65 ± 13 | 74 ± 21 | 0.04 |
| Low-density lipoprotein cholesterol (mg/dl) | 97 ± 27 | 87 ± 28 | 104 ± 25 | 0.01 |
| Triglycerides (mg/dl) | 77 ± 34 | 82 ± 41 | 75 ± 29 | 0.45 |
| Hemoglobin (g/dL) | 14.4 ± 1.1 | 14.5 ± 1.3 | 14.4 ± 1.0 | 0.73 |
| Hematocrit (%) | 41.8 ± 3.2 | 41.8 ± 3.7 | 41.8 ± 2.9 | 0.96 |
| Albumin (g/dL) | 4.5 ± 0.2 | 4.5 ± 0.2 | 4.6 ± 0.2 | 0.02 |
| Total bilirubin (mg/dL) | 0.83 ± 0.41 | 0.84 ± 0.45 | 0.82 ± 0.39 | 0.90 |
| Direct bilirubin (mg/dL) | 0.17 ± 0.08 | 0.18 ± 0.09 | 0.16 ± 0.07 | 0.34 |
| Alkaline phosphatase (IU/L) | 56.7 ± 16.6 | 58 ± 16 | 56 ± 17 | 0.50 |
| Alanine aminotransferase (IU/L) | 24.3 ± 14.3 | 28 ± 18 | 22 ± 11 | 0.07 |
| Aspartate aminotransferase (IU/L) | 27.9 ± 22.7 | 28 ± 12 | 28 ± 28 | 0.87 |
| Statin (Dose in mg) | Number of Runners |
|---|---|
| Fluvastatin | |
| 80 | 1 |
| Atorvastatin | |
| 5 | 2 |
| 10 | 2 |
| 20 | 5 |
| 80 | 1 |
| Rosuvastatin | |
| 10 | 2 |
| Simvastatin | |
| 10 | 2 |
| 20 | 5 |
| 40 | 5 |
| Lovastatin | |
| 20 | 2 |
| Pravastatin | |
| 10 | 2 |
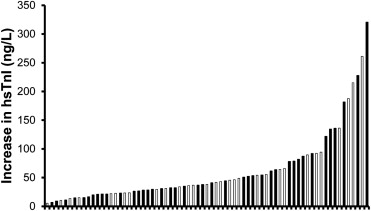
cTnI could be detected in 13% and 99% of the runners before the race via the contemporary cTnI and hsTnI assay, respectively (p <0.001), with an average of 7% of the participants exceeding the 99th percentile value with both assays ( Table 3 ). Contemporary cTnI and hsTnI concentrations were increased at FINISH (both p <0.001), with detectable cTnI concentrations in 78% and 100% for the 2 assays, respectively (p <0.001). Notably, 100% of participants demonstrated an increase in hsTnI concentrations ( Figure 1 ), with 47% and 54% of the runners exceeding the 99th percentile for the contemporary cTnI and hsTnI assays, respectively (p = 0.40). Contemporary cTnI and hsTnI concentrations were highly correlated at FINISH (r 2 = 0.96, p <0.001; Figure 2 ). cTnI concentrations remained increased at POST-24H and detectable in 52% and 100% of the participants with the contemporary cTnI and hsTnI assay, respectively (p <0.001). At POST-24H, the number of participants exceeding the 99th percentile was comparable for the contemporary cTnI (25%) and hsTnI (27%) assay (p = 0.85). The exercise-induced changes in CK concentrations in statin-using and nonstatin-using runners were published previously. NT-proBNP (p <0.001) myoglobin (p <0.001), and cystatin C concentrations (p <0.001) also were significantly increased at FINISH and POST-24H.
| Variable | Conventional cTnI Assay | High Sensitive cTnI Assay | ||
|---|---|---|---|---|
| Statin | Control | Statin | Control | |
| cTnI concentration >LOD | ||||
| PRE | 7% | 17% | 100% | 98% |
| FINISH | 87% | 71% | 100% | 100% |
| POST-24 hours | 53% | 56% | 100% | 100% |
| cTnI concentration >99th percentile | ||||
| PRE | 3% | 10% | 3% | 10% |
| FINISH | 47% | 46% | 57% | 51% |
| POST-24 hours | 20% | 29% | 30% | 24% |

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


