Coronary artery calcium (CAC) is a marker of atherosclerosis, and CAC progression is independently associated with all-cause mortality in the general population but not convincingly in subjects with diabetes mellitus (DM). The aim of this study was to ascertain the differences in the rates of CAC progression, the effect of statin therapy, and all-cause mortality in subjects with and without DM. The study group consisted of 296 asymptomatic subjects with type 2 DM and 300 controls (mean age 59 ± 6 years, 29% women) who underwent baseline and follow-up CAC scans within a 2-year interval. Absolute annual CAC score change, percentage annual CAC progression(ΔCAC%), event-free survival, and the effect of statin therapy on survival were all assessed. The mean follow-up duration was 56 ± 11 months. Absolute annual CAC score change was 81 ± 10 in subjects with DM and 34 ± 5 in controls (p = 0.0001). Percentage annual CAC progression was 29 ± 9% in subjects with DM and 10 ± 7% in controls (p = 0.0001). The hazard ratios of death in 3 groups of subjects with DM compared to controls without DM were 1.88 (95% confidence interval [CI] 1.51 to 2.36, p = 0.0001) for ΔCAC of 10% to 20%, 2.29 (95% CI 1.56 to 3.38, p = 0.0001) for ΔCAC of 21% to 30%, and 6.95 (95% CI 2.23 to 11.53, p = 0.0001) for ΔCAC >30%, all compared to ΔCAC <10%. The adjusted hazard ratios of all-cause mortality in subjects receiving compared to those not receiving statin therapy were 0.29 (95% CI 0.13 to 0.56, p = 0.001) in those without DM and without CAC progression, 0.51 (95% CI 0.21 to 0.73, p = 0.001) in those with DM and without CAC progression, and 0.71 (95% CI 0.25 to 0.91, p = 0.003) in those without DM and with CAC progression, with all 3 groups compared to 1.0 (reference) in those with DM, with CAC progression and without statin therapy. In conclusion, CAC progression was greater and event-free survival lower in patients with DM compared to controls in proportion to the extent of CAC progression. These results suggest that CAC progression is an independent predictor of all-cause mortality in patients with DM.
Coronary artery disease (CAD) is the most common cause of death in patients with diabetes mellitus (DM). In fact, >70% of patients with type 2 DM die of cardiovascular diseases. All manifestations of cardiovascular disease, including stroke, myocardial infarction, sudden cardiac death, and angina pectoris, are >2 times more common in patients with type 2 DM compared to the general population. Coronary artery calcium (CAC) measured by computed tomography is a dependable marker of coronary artery atherosclerosis. CAC score is known to be an excellent predictor of cardiovascular disease and events in patients both with and without DM. This study was designed to evaluate the rates of CAC progression and the effect of statin therapy in patients with DM compared to controls without DM. The influence of statin therapy was addressed in this study primarily to improve the current clinical practice of statin use and increase its adherence. Furthermore, we aimed to assess the rate of CAC progression as an independent predictor of all-cause mortality in patients with DM.
Methods
The study sample consisted of 596 asymptomatic subjects (296 with type 2 DM and 300 matched controls, mean age 59 ± 6 years, 29% women) referred by primary care physicians for CAC imaging. In this retrospective study, the asymptomatic control subjects were matched to the patients with DM with respect to age, gender, CAD risk factors, and baseline CAC score. CAD risk factors included current cigarette smoking, history of premature CAD in a first-degree relative (men aged ≤55 years and women aged ≤65 years), hypertension, hypercholesterolemia, and the use of statin therapy ( Table 1 ). Subjects underwent baseline and follow-up cardiac computed tomographic examinations with a maximum interval between scans of 2 years (range 1 to 2) for the purpose of remeasuring CAC scores. All study subjects were asked to complete a questionnaire at the time of the initial and follow-up scans to assess demographics, ethnicity, and cardiovascular risk factors. The questionnaires collected information on cardiac risk factors, as well as lifestyle, diet type, alcohol intake, and histories of cardiovascular, pulmonary, and renal diseases. The follow-up questionnaire at the time of the second CAC scan collected information on interim changes since the time of the initial cardiac computed tomographic scan. Information acquired included major adverse events (nonfatal myocardial infarction, unstable angina. and stroke) and pertinent changes in antihypertensive, antidiabetic, and cholesterol-lowering medications. Additional information was elicited regarding interim cardiac diagnostic tests, changes in diet, lifestyle, exercise level, tobacco smoking habits, alcohol consumption, antiplatelet therapy, and vitamin supplements. Information about menopausal status and the use of oral contraceptives or hormone replacement therapy in women was ascertained.
| Variable | Matched Controls † | Patients With DM | p Value |
|---|---|---|---|
| (n = 300) | (n = 296) | ||
| Age (yrs) | 59 ± 6 | 59 ± 6 | — |
| Women | 29% (87) | 29% (86) | — |
| Baseline CAC score | 276 ± 41 | 291 ± 49 | 0.9 |
| Statin therapy | 50% (150) | 55% (163) | 0.8 |
| Hypertension | 26% (78) | 68% (201) | 0.03 |
| Family history of premature CAD § | 50% (150) | 40% (118) | 0.7 |
| Current tobacco smokers | 16% (48) | 19% (56) | 0.6 |
| Absolute annual CAC score change | 34.3 ± 4.8 | 80.6 ± 10 | 0.0001 |
| ΔCAC% ∗ | 10.2 ± 6.7 | 29.4 ± 8.7 | 0.0001 |
| CAC progressors ‡ | 33.6% (101) | 62.5% (185) | 0.0001 |
∗ CAC Progression (ΔCAC%) = (Annual change in CAC/baseline CAC) × 100.
† Matched for age, gender, cardiovascular disease risk, and baseline CAC.
§ CAD in a first-degree relative (men aged ≤55 years, women aged ≤65 years).
All study participants agreed to and underwent 2 serial noncontrast CAC scans using an Imatron C-150XL Ultrafast computed tomographic scanner (GE-Imatron, San Francisco, California). All patients were informed about the risks and benefits of each imaging study, and they all demonstrated understanding and agreed to proceed. This retrospective study was approved by the local institutional review board. Contiguous tomographic images were obtained at 3-mm intervals starting 1 cm below the carina and progressing inferiorly to capture the complete coronary tree.
CAC scores were calculated using the Agatston method, which involves the multiplication of the area of a calcified focus by a cofactor based on the peak density of the lesion. The total CAC score was calculated by adding the individual scores of all lesions found along the entire coronary artery tree. All-cause mortality was assessed after a mean follow-up period of 56 ± 11 months from the time of the second CAC scan (range 0.7 to 14.5 years). Event-free survival was ascertained for all subjects. Data on mortality were obtained in 100% of the study subjects. Epidemiologic methods for data collection included inquiry of events by researchers blinded to historical and CAC results. The mortality status of all subjects was verified using the Social Security Death Index ( http://www.ntis.gov ) .
We calculated the absolute annual CAC score change and the percentage annual CAC progression (ΔCAC%) ([annual change in CAC/baseline CAC] × 100) for all patients and controls ( Table 1 ). The control subjects were matched to the DM group for age, gender, CAD risk factors (as reported earlier), and baseline CAC score. A p value <0.05 was considered statistically significant.
Multivariate Cox proportional-hazards models were developed to predict all-cause mortality. Hazard ratios of death and 95% confidence intervals (CIs) across the 2 groups were assessed by means of Cox proportional-hazards regression analysis models. Analysis models were all adjusted for age, gender, all CAD risk factors, and baseline CAC score.
Hazard ratios of death and 95% CIs were computed using 3 models on the basis of categories of ΔCAC% as a marker of the percentage annual rate of CAC progression. For the purpose of differentiating the levels of CAC progression, 3 models were designed to evaluate the rates of CAC progression in the DM group compared to the matched control group ( Table 2 ). Models 1, 2, and 3 characterized mild, moderate, and severe percentage annual rates of CAC progression, respectively. Model 1 was characterized as ΔCAC of 10% to 20% versus ΔCAC <10%, model 2 as ΔCAC of 21% to 30% versus ΔCAC <10%, and model 3 as ΔCAC >30% versus ΔCAC <10%. In addition, event-free survival was calculated on the basis of a mean follow-up period of 56 ± 11 months from the time of the second noncontrast computed tomography scan for 3 models on the basis of the extent of CAC progression. For this study, an annual CAC progression of >15% was considered evidence of true progression and not due to measurement error and interscan variability. Furthermore, the use and survival benefit of statins were also evaluated and compared for all patients in the DM and control groups, both with and without CAC progression.
| Model | ΔCAC% | Matched Controls | Patients With DM | p Value |
|---|---|---|---|---|
| 1 | 10%–20% vs <10% | 1.0 (reference) | 1.88 (1.51–2.36) | 0.0001 |
| 2 | 21%–30% vs <10% | 1.0 (reference) | 2.29 (1.56–3.38) | 0.0001 |
| 3 | >30% vs <10% | 1.0 (reference) | 6.95 (2.23–11.53) | 0.0001 |
Results
The mean baseline CAC scores for the DM group (n = 296) and matched control group (n = 300) were 291 ± 49 and 276 ± 41, respectively ( Table 1 ). Absolute annual CAC score change was 34.3 ± 4.8 in matched controls and 80.6 ± 10 in the DM group (p = 0.0001). ΔCAC% was 10.2 ± 6.7% in matched controls and 29.4 ± 8.7% in the DM group (p = 0.0001).
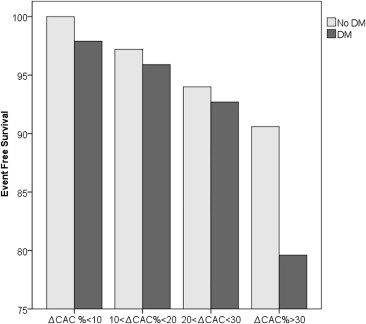
Hazard ratios of death in patients with DM compared to matched controls were 1.88 (95% CI 1.51 to 2.36, p = 0.0001) for model 1, 2.29 (95% CI 1.56 to 3.38, p = 0.0001) for model 2, and 6.95 (95% CI 2.23 to 11.53, p = 0.0001) for model 3, with all 3 models compared to ΔCAC <10%, independent of age, gender, and CAD risk factors ( Table 2 ). After a mean period of 56 ± 11 months from the second scan, event-free survival was evaluated for 4 models on the basis of the extent of rate of CAC progression ( Table 3 ). Event-free survival was lower in the DM subgroups compared to the control subgroups, and the decrease was proportional to the rate of CAC progression ( Figure 1 ). Kaplan-Meier curves of event-free survival also show the separate relation of mortality and CAC progression on the basis of 4 models among the controls ( Figure 2 ) and the patients with DM ( Figure 3 ).
| Variable (ΔCAC) | DM | No DM | p Value |
|---|---|---|---|
| <10% | 97.9% | 100% | 0.50 |
| 10%–20% | 95.9% | 97.2% | 0.01 |
| 21%–30% | 92.7% | 94% | 0.01 |
| >30% | 79.6% | 90.6% | 0.0001 |

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


