In the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) study, a revascularization strategy trial with optimal medical therapy in both arms, the low-density lipoprotein (LDL) cholesterol goal was 60 to 85 mg/dl; this was revised to <70 mg/dl in 2004. COURAGE patients (n = 2,287) were titrated with increasing statin doses to achieve the initial LDL cholesterol goal using a prespecified protocol. Ezetimibe was not available when study enrollment began in 1999 but became available after approval in 2003. After maximizing statin dose, ezetimibe was added to reach the LDL cholesterol goal in 34% of patients (n = 734). Median baseline LDL cholesterol was higher in patients who received ezetimibe than in those who did not (109 vs 96 mg/dl). At baseline, 18% of patients who would later receive ezetimibe had LDL cholesterol <85 mg/dl, and 8% had LDL cholesterol <70 mg/dl. On maximum tolerated statin (with or without other lipid-lowering drugs), 40% had LDL cholesterol <85 mg/dl and 23% had LDL cholesterol <70 mg/dl before starting ezetimibe. At the final study visit, 68% of ezetimibe patients achieved LDL cholesterol <85 mg/dl, and 46% achieved LDL cholesterol <70 mg/dl. Using Cox regression analysis, the most significant factors associated with achieving LDL cholesterol goals were lower baseline LDL cholesterol, average statin dose, and ezetimibe use. In conclusion, after maximizing statin dose, the addition of ezetimibe results in a substantial increase in the percentage of patients who reach LDL cholesterol goal, a key component of optimal medical therapy.
The Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial compared the effectiveness of intensive lifestyle and pharmacologic interventions (optimal medical therapy) with or without percutaneous coronary intervention in 2,287 patients with stable coronary artery disease. The 4.6-year cumulative primary event rates were 19.0% in the percutaneous coronary intervention group and 18.5% in the optimal medical therapy group (hazard ratio for the percutaneous coronary intervention group 1.05, 95% confidence interval 0.87 to 1.27, p = 0.62). The cornerstone of optimal medical therapy in COURAGE was aggressive therapy to lower low-density lipoprotein (LDL) cholesterol to a prespecified target. In this report, we describe in detail the results of the lipid intervention in COURAGE, with a focus on the impact of the addition of ezetimibe to simvastatin.
Methods
The methods used in COURAGE ( ClinicalTrials.gov identifier NCT00007657 ) have been reported previously. The study protocol was approved by the human rights committee at the coordinating center and by local institutional review boards. An independent data and safety monitoring board monitored the trial. Data management and analyses were performed solely by the data coordinating center and overseen by the trial’s executive committee, which had full access to the data and analyses and vouches for their accuracy and completeness.
The primary lipid goal was to achieve LDL cholesterol of 60 to 85 mg/dl. This target was established by the steering committee in 1997, when the National Cholesterol Education Program goal for patients with coronary artery disease was <100 mg/dl, and the safety of lowering LDL cholesterol to <70 mg/dl with pharmacologic therapy was unknown. In July 2004, the National Cholesterol Education Program established an optional LDL cholesterol goal of <70 mg/dl for “very high risk patients,” and the COURAGE steering committee adopted that as the primary lipid goal for all COURAGE patients for the remainder of the trial. Secondary lipid and lipoprotein goals throughout the trial were triglycerides <150 mg/dl and high-density lipoprotein (HDL) cholesterol >40 mg/dl.
Each patient met with a nurse case manager at baseline; at 1, 2, 3, and 6 months; and then every 6 months until the study ended. At each visit, lifestyle and medication adherence were assessed, and fasting lipid values were measured. Lipids and lipoproteins were analyzed at a core laboratory (Washington University, St. Louis, Missouri). Lifestyle intervention was delivered equally to both treatment groups at each visit. Case managers were trained to provide brief, practical, focused behavioral interventions for smoking cessation, nutrition, physical activity, and weight management.
Diet was assessed using the MEDFICTS questionnaire. Nutritional counseling was designed to achieve the dietary guidelines of the National Cholesterol Education Program.
Pharmacologic therapy conformed to established guidelines for secondary prevention. All patients received the same medical therapy for secondary prevention regardless of randomized treatment assignment. Simvastatin was provided free of charge to study patients by Merck & Company (Whitehouse Station, New Jersey), on the condition that the drug be prescribed according to approved labeling instructions. A lipid treatment algorithm for initiating and titrating therapy was provided to all site investigators and study coordinators, and this was updated periodically as dosing instructions in the package insert changed and as new drugs became available. Accordingly, patients recruited in 1999 were started on simvastatin 20 mg regardless of baseline LDL cholesterol. In 2001, patients entering the trial were started on simvastatin 40 mg. If patients were already taking statins when they entered the study, they were switched to equivalent doses of simvastatin (if >20 mg) according to a statin equivalency table prepared by the steering committee (see the Appendix ). Extended-release niacin was donated by Kos Pharmaceuticals (Cranbury, New Jersey) in 2001. When ezetimibe became available in the United States and Canada in 2003, it became the primary add-on therapy to simvastatin when needed to achieve the LDL cholesterol goal. At each visit, the dose of each lipid medication taken by each patient was recorded. If simvastatin was not tolerated or was not effective, patients were allowed to use other statins. After the prevailing LDL cholesterol goal was achieved, an attempt was made to increase HDL cholesterol to >40 mg/dl and to lower triglycerides to <150 mg/dl with lifestyle change, extended-release niacin, fibrates, and omega-3 fatty acids. Patients received lipid medications at no cost to them, except for fenofibrate and omega-3 fatty acids.
Results are generally presented for the entire COURAGE patient cohort in aggregate because previous reports showed no differences between randomized treatment groups in lipid and lipoprotein goals achieved. Median values are used throughout for lipoproteins because the distributions were skewed. For the standard errors of the medians, we used the approximation based on the interquartile range. Most of the patients took simvastatin throughout the trial, but for those taking different statins, for the purposes of analysis, the dose was translated into a simvastatin equivalent (see the Appendix ). We used <100, <85, and <70 mg/dl as goals for LDL cholesterol in the analysis. Although <100 mg/dl was not a specific study goal, we used it in our analysis because it is the minimum goal recommended by the National Cholesterol Education Program for secondary prevention. The time to each LDL cholesterol goal was analyzed using Cox regression methods. The variables included were baseline lipoproteins as well as average statin dose for each patient and the time-dependent variable of first use of ezetimibe. Average statin dose was calculated as the sum of (dose × time on each dose)/total time.
Results
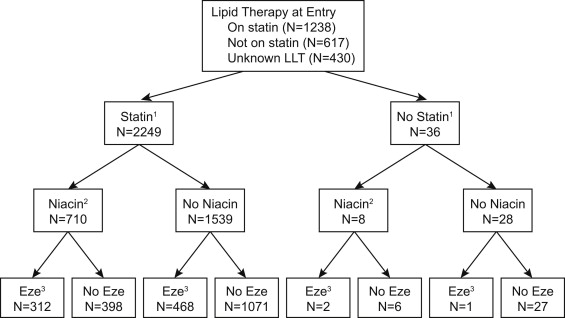
Figure 1 shows patient flow during the trial. Patients who never took statins or ezetimibe before or during the trial tended to be older, were less likely to smoke, and had lower diastolic blood pressures ( Table 1 ). In addition, those patients who were not prescribed ezetimibe during the trial had lower median LDL cholesterol at baseline.
| Variable | All Patients | Ever on Statin During Follow-Up | Never on Statin | p Value | Ever on Ezetimibe During Follow-Up | Never on Ezetimibe | p Value |
|---|---|---|---|---|---|---|---|
| Number of patients | 2,285 | 2,249 (98%) | 36 (2%) | — | 783 (34%) | 1,502 (66%) | — |
| Age (yrs) | 62 ± 10 | 62 ± 10 | 68 ± 7 | <0.001 | 59 ± 10 | 64 ± 10 | <0.001 |
| Men | 1,947 (85%) | 1,920 (850%) | 27 (75%) | 0.08 | 667 (85%) | 1,280 (85%) | 0.98 |
| White race | 1,963 (86%) | 1,933 (86%) | 30 (83%) | 0.65 | 670 (86%) | 1,293 (86%) | 0.74 |
| Smokers | 653 (29%) | 647 (29%) | 6 (17%) | 0.11 | 242 (31%) | 411 (27%) | 0.07 |
| Body mass index (kg/m 2 ) | 30 ± 5 | 30 ± 5 | 29 ± 5 | 0.40 | 30 ± 5 | 29 ± 5 | 0.006 |
| Systolic blood pressure (mm Hg) | 133 ± 20 | 132 ± 19 | 135 ± 23 | 0.45 | 131 ± 18 | 133 ± 20 | 0.04 |
| Diastolic blood pressure (mm Hg) | 74 ± 11 | 74 ± 11 | 69 ± 14 | 0.04 | 75 ± 11 | 74 ± 11 | <0.001 |
| Diabetes | 766 (34%) | 754 (34%) | 12 (33%) | 0.92 | 230 (30%) | 544 (36%) | 0.003 |
| Median ± SE LDL cholesterol (mg/dl) | 101 ± 0.8 | 101 ± 0.8 | 91 ± 9 | 0.13 | 109 ± 1.4 | 96 ± 1.0 | <0.001 |
| Median ± SE HDL cholesterol (mg/dl) | 39 ± 0.3 | 39 ± 0.3 | 40 ± 1.8 | 0.75 | 40 ± 0.4 | 39 ± 0.3 | 0.04 |
| Median ± SE triglycerides (mg/dl) | 146 ± 2.1 | 146 ± 2.1 | 119 ± 19 | 0.31 | 152 ± 3.6 | 143 ± 2.6 | 0.05 |
Table 2 lists baseline and on-trial lipid and lipoprotein values and frequency of lipid medication use during the trial. Values for the 2 treatment groups were very similar and validate the aggregation of the 2 treatments in the remaining analyses. At baseline, among patients whose medications before study entry were known, 67% were taking statins, and by 5 years, this proportion had increased to 93%. The percentage of patients using ezetimibe increased from <1% at baseline to 31% at 5 years. The percentages of patients at baseline taking simvastatin 20 mg decreased during 5 years from 38% to 18%, of those taking 40 mg decreased from 41% to 35%, and of those taking 80 mg increased from 9% to 38% at 5 years. The mean average statin dose for each patient was 40 ± 23 mg. Twelve percent of patients took statins other than simvastatin. Because of adverse effects or patient preference, 2% to 4% of patients took no lipid-lowering medications at all, and an additional 2% to 5% of patients took only nonstatin lipid-lowering medications. LDL cholesterol values decreased progressively during the trial and were associated temporally with the prespecified titration algorithm, the availability of ezetimibe, and the revision of the LDL cholesterol goal from 60 to 85 mg/dl to <70 mg/dl.
| Variable | All Patients | PCI Plus OMT | OMT | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline ∗ | 1 yr | 3 yrs | 5 yrs | Baseline ∗ | 1 yr | 3 yrs | 5 yrs | Baseline ∗ | 1 yr | 3 yrs | 5 yrs | |
| Lipids | ||||||||||||
| n | 2,287 | 2,071 | 1,678 | 841 | 1,148 | 1,031 | 820 | 423 | 1,137 | 1,010 | 824 | 406 |
| Total cholesterol (mg/dl) | 174 ± 1.0 | 153 ± 0.8 | 147 ± 0.9 | 142 ± 1.2 | 172 ± 1.3 | 156 ± 1.2 | 148 ± 1.2 | 144 ± 1.7 | 177 ± 1.4 | 150 ± 1.1 | 145 ± 1.3 | 141 ± 1.7 |
| HDL cholesterol (mg/dl) | 39 ± 0.3 | 41 ± 0.3 | 42 ± 0.3 | 41 ± 0.5 | 39 ± 0.4 | 42 ± 0.4 | 43 ± 0.5 | 41 ± 0.7 | 39 ± 0.4 | 41 ± 0.4 | 42 ± 0.5 | 41 ± 0.8 |
| LDL cholesterol (mg/dl) | 101 ± 0.8 | 82 ± 0.7 | 75 ± 0.7 | 72 ± 0.9 | 100 ± 1.2 | 84 ± 1.0 | 76 ± 0.9 | 71 ± 1.3 | 102 ± 1.2 | 81 ± 0.9 | 74 ± 0.9 | 72 ± 1.2 |
| Triglycerides (mg/dl) | 146 ± 2.1 | 131 ± 2.0 | 124 ± 2.0 | 126 ± 3.1 | 143 ± 3.0 | 129 ± 2.7 | 123 ± 2.8 | 123 ± 4.1 | 150 ± 3.0 | 133 ± 2.9 | 125 ± 2.8 | 132 ± 4.6 |
| Medications | ||||||||||||
| n | 1,855 | 2,073 | 1,676 | 846 | 934 | 1,044 | 837 | 428 | 921 | 1,029 | 839 | 418 |
| Statin | 1,239 (67%) | 1,945 (94%) | 1,550 (92%) | 785 (93%) | 622 (67%) | 972 (93%) | 780 (93%) | 398 (93%) | 617 (67%) | 973 (95%) | 770 (92%) | 387 (93%) |
| Ezetimibe | 0 (0%) | 74 (4%) | 286 (17%) | 267 (31%) | 0 (0%) | 38 (4%) | 152 (18%) | 134 (31%) | 0 (0%) | 36 (3%) | 134 (16%) | 133 (32%) |
| Niacin | 23 (1%) | 318 (15%) | 329 (20%) | 187 (22%) | 11 (1%) | 154 (15%) | 174 (21%) | 92 (21) | 12 (1%) | 164 (16%) | 155 (18%) | 95 (23%) |
| Resin | 2 (<1%) | 12 (1%) | 10 (1%) | 2 (<1%) | 0 (0%) | 6 (1%) | 5 (1%) | 0 (0%) | 2 (<1%) | 6 (1%) | 5 (1%) | 2 (<1%) |
| Fibrates | 63 (3%) | 131 (6%) | 119 (7%) | 58 (7%) | 32 (3%) | 60 (6%) | 45 (5%) | 23 (5%) | 31 (3%) | 71 (7%) | 74 (9%) | 35 (8%) |
Table 3 lists the changes from baseline in lipid and lipoprotein values for different lipid-lowering drug regimens. Figure 2 shows the proportions of patients at 3 different LDL cholesterol goals (<100, <85 and <70 mg/dl) at baseline and last visit for patients who did and did not take ezetimibe. Despite a lower proportion of patients who would later receive ezetimibe being at goal at baseline (p <0.001 for all 3 targets), at their last visits, LDL cholesterol goal attainment for patients who received ezetimibe was similar to that of those who did not (p = 0.60, p = 0.90, and p = 0.30, respectively). This finding was due to a greater reduction in LDL cholesterol in patients who received ezetimibe compared with those who did not. The proportions of patients at goal immediately before starting ezetimibe and during ezetimibe therapy are shown in Figure 2 . In aggregate (i.e., combining all COURAGE patients) at baseline, 49% had LDL cholesterol <100 mg/dl, 29% had LDL cholesterol <85 mg/dl, and 14% had LDL cholesterol <70 mg/dl. After titrating statin therapy to the maximum tolerated dose (with or without other lipid-lowering drugs) but before adding ezetimibe, those numbers increased to 66% with LDL cholesterol <100 mg/dl, 40% with LDL cholesterol <85 mg/dl, and 23% with LDL cholesterol <70 mg/dl. After adding ezetimibe, the percentages at goal increased further to 83% to 85% with LDL cholesterol <100 mg/dl, 70% to 73% with LDL cholesterol <85 mg/dl, and 45% to 47% with LDL cholesterol <70 mg/dl over the following 6 to 30 months ( Figure 3 ). In aggregate at their last visits, 83% of patients taking ezetimibe had LDL cholesterol <100 mg/dl, 69% had LDL cholesterol <85 mg/dl, and 44% had LDL cholesterol <70 mg/dl. Median HDL cholesterol was 38.0 mg/dl for men and 47.6 mg/dl for women. During the trial, HDL cholesterol increased as much as 3 mg/dl on average, with no significant differences in the increase of HDL cholesterol between the genders (data not shown).
| Therapy | Lipid (mg/dl) | 1 yr | 3 yrs | 5 yrs | |||
|---|---|---|---|---|---|---|---|
| n | Median ± SE | n | Median ± SE | n | Median ± SE | ||
| All patients | LDL cholesterol | 1,980 | −18 ± 0.9 | 1,617 | −24 ± 1.0 | 804 | −29 ± 1.6 |
| HDL cholesterol | 2,014 | 2.0 ± 0.2 | 1,640 | 3.0 ± 0.3 | 812 | 1.6 ± 0.4 | |
| Triglycerides | 2,018 | −14 ± 1.7 | 1,640 | −19 ± 1.9 | 813 | −24 ± 3.1 | |
| Receiving statin monotherapy | LDL cholesterol | 1,431 | −17 ± 1.0 | 923 | −23 ± 1.3 | 364 | −26 ± 2.2 |
| HDL cholesterol | 1,446 | 2.0 ± 0.2 | 932 | 3.3 ± 0.3 | 365 | 1.6 ± 0.6 | |
| Triglycerides | 1,447 | −12 ± 1.9 | 930 | −15 ± 2.3 | 365 | −12 ± 4.1 | |
| Receiving statin + ezetimibe ± other therapy | LDL cholesterol | 67 | −26 ± 5.3 | 253 | −32 ± 2.8 | 240 | −43 ± 2.9 |
| HDL cholesterol | 67 | 1.2 ± 1.0 | 258 | 2.0 ± 0.6 | 242 | 0.9 ± 0.7 | |
| Triglycerides | 67 | −28 ± 7.9 | 259 | −25 ± 4.8 | 243 | −27 ± 5.1 | |
| Receiving nonstatin lipid-lowering therapy | LDL cholesterol | 34 | −6.5 ± 9.4 | 45 | −10 ± 7.2 | 28 | −21 ± 9.5 |
| HDL cholesterol | 36 | 1.3 ± 1.7 | 46 | 3.7 ± 1.4 | 30 | 3.1 ± 2.4 | |
| Triglycerides | 36 | −23 ± 18 | 46 | −13 ± 13 | 30 | −24 ± 13 | |
| Not receiving any lipid-lowering therapy | LDL cholesterol | 83 | −2.0 ± 5.4 | 73 | −11 ± 3.7 | 24 | −13 ± 9 |
| HDL cholesterol | 85 | −1.2 ± 1.0 | 73 | −0.8 ± 0.9 | 25 | −1.0 ± 1.2 | |
| Triglycerides | 85 | −4.4 ± 7.6 | 74 | 3.6 ± 7.2 | 25 | −10 ± 14 | |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


