Number of patients
63
Age at treatment onset; mean ± SD, range (years)
10.5 ± 4.3, 0.5–17.5
Gender; males/females
36/27
Etiology, number of patients
Idiopathic factors
45 (71.4 %)
Toxic exposure
6 (9.5 %)
Seronegative hepatitis
4 (6.4 %)
Hepatitis B Virus (HBV)
2 (3.2 %)
Hepatitis C Virus (HCV)
3 (4.7 %)
Cytomegalovirus (CMV)
1 (1.5 %)
Parvovirus B19
2 (3.2 %)
Staging of aplastic anemia
Severe aplastic anemia
54 (85.7)
Very severe aplastic anemia
9 (14.3)
2.2 Protocol
All enrolled patients received r-ATG (Lymphoglobulin, Genzyme; Cambridge, USA) intravenously in a dose of 3.75 mg/kg body weight on Days 1–5 and cyclosporine-A (Sandimmun Neoral Novartis; Pharma GmbH, Nurnberg; Germany) orally in a dose of 5 mg/kg body weight over Days 1–180. The dose of cyclosporine was modified according to its serum concentration to keep it in a range of 100–200 ng/ml. Granulocyte colony-stimulating factor (Fligrastim-Neupogen, Amgen; Thousand Oaks; USA) was given subcutaneously or intravenously only to patients with severe infections non-responding to antibiotics and antifungal medications. The presence of remission was evaluated at Days 112, 180, and 360 from the onset of treatment. Complete remission was defined as the absolute neutrophil count >1.5 × 109/L, platelets >100 × 109/L, and hemoglobin concentration >11.0 g/L. Partial remission was diagnosed when the neutrophil count was >0.5 × 109/L, platelets >20 × 109/L, and hemoglobin concentration >8.0/L.
2.3 Statistical Analysis
The Kaplan-Meier estimator was used to draw survival curves and curves showing the time of disease progression and relapse. Overall survival, expressed in years, was defined as the interval between the diagnosis and death of a patient. The event free survival, expressed in years, was defined as the time from the diagnosis to death, disease relapse, or the last follow-up. The time to disease relapse was considered as the number of years between the diagnosis of AA and its relapse. If no demise or relapse occurred, the last follow-up with the evaluation of treatment response, was considered as right-censoring. The longest follow-up period equaled 10 years. All statistical tests carried out during the analysis considered a p-value of 0.05 and confidence intervals that did not include 1 as defining significance. Statistical analysis was carried out with a commercial package Stata ver. 11.0.
3 Results
3.1 Response to Immunosuppressive Treatment
At Day 112, complete remission occurred in only 10 patients (15.9 %) and partial by 18 (28.5 %); in total in 28 out of the 63 treated patients (44.4 %) (Table 2). At Day 180, complete remission occurred in 15 patients (23.8 %) and partial in 16 (25.4 %); in total in 31 patients (49.2 %). One year after the onset of therapy, complete remission occurred in 24 patients (38.0 %) and partial in 10 (15.9 %); in total in 34 patients (64.9 %).
Table 2
Response to rabbit antithymocyte globulin (r-ATG)
Day 112 | Day 180 | Day 360 | |
|---|---|---|---|
CR | 10 | 15 | 24 |
PR | 18 | 16 | 10 |
NR | 28 | 23 | 14 |
Death | 10 | 12 | 15 |
The response to treatment in relation to the disease severity at Day 360 is as follows: in the group of nine children with very severe AA, complete remission occurred in one child (11.1 %), partial in three (3.3 %), and five children (55.5 %) did not presented any response. In total, therapy was effective to some extend in 4 patients (44.4 %). On the other hand, 30 patients out of the 54 children with severe AA responded to treatment; complete remission was observed in 23 (42.5 %) and partial PR in 7 (13.0 %). The group of patients with severe AA had better treatment results in comparison with the children with very severe AA (p = 0.003). Remission was not achieved in 14 out of the 63 children (22.2 %) after 1 year from the onset of therapy.
The treatment course with r-ATG was repeated in four cases and three (75.0 %) of them have remained in complete remission until today. Hematopoietic stem cell transplantation from unrelated donors was carried out in 11 patients out of the 15 non-responders.
3.2 Relapses
Severe aplastic anemia relapsed in four patients (6.3 %) out of the 63 children treated with r-ATG. In two of them the course of r-ATG was repeated and one underwent hematopoietic stem cell transplantation from unrelated donors. The estimated probability of no relapse was 98 % after the second, third, fourth, and fifth year of follow-up (95%CI 0.86; 0.99). The estimated probability of no relapse during the next 6–10 years was 93 % (95%CI 0.72; 0.98).
3.3 Clonal Transformation
One child, 5 years after achieving remission, was diagnosed with paroxysmal nocturnal hemoglobinuria. Secondary myelodysplastic syndrome or acute myeloblastic leukemia were not observed in the study group.
3.4 Survival Analysis
Fifteen deaths (23.8 %) out of the 63 patients, including 5 early demises (up to Day 90 from treatment onset) and 10 late (after Day 90) were observed in the study group. The major causes of death were severe infections (7 cases) and cerebral hemorrhage (2 cases) (Table 3).
Table 3
Causes of deaths over the treatment course
Before Day 90 | After Day 90 | |
|---|---|---|
Infectious causes: | 2 | 5 |
E. coli, | 1 | 2 |
S. aureus | ||
E. cloacae, | 1 | |
E. faecium, | 1 | |
P. aeruginosa, | 1 | |
K. pneumoniae | 1 | |
Cerebral hemorrhage | 1 | 1 |
Others: | ||
Pancreatitis | 1 | |
Pneumonia & pleural empyema | 1 | |
Cerebral mycosis | 1 | |
Serum sickness | 1 | |
Unknown | 3 | |
All | 5 | 10 |
The estimated 10-year overall survival rate and 10-year event-free survival rate were 67 % and 57 %, respectively. The effectiveness of the first-line treatment with r-ATG was inferior to horse antithymocyte globulin. While analyzing the overall survival rate in the whole group of severe AA patients (n = 63) treated with r-ATG, it can be concluded that the estimated survival probability was 80 % (95%CI 0.68; 0.88) 2 years after the onset. However, the overall survival rate was 67 % (95%CI 0.51; 0.80) in the ninth and tenth year of follow-up (Fig. 1a).
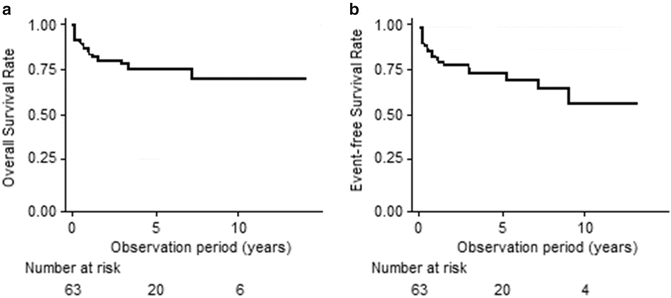





Fig. 1
Overall survival rate (Panel A) and event-free survival rate (Panel B) in children treated with rabbit antithymocyte globulin (r-ATG)
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


