Immunocompromised Host
Introduction
Infection is the main pulmonary complication and the commonest cause of radiographic abnormality in immunocompromised non–acquired immunodeficiency syndrome (AIDS) patients (1,2,3,4,5,6,7). The number of these patients has increased considerably in the last two decades because of greater number of hematopoietic stem cell (bone marrow) and solid organ transplantations, advances in the treatment of cancer, and increased use of immunosuppressive therapy in a number of other conditions (8,9,10,11,12,13,14,15,16). A wide variety of pulmonary infections may occur in these patients and result in considerable morbidity and mortality (17,18,19,20,21,22).
Immunocompromised non–AIDS patients are at risk not only for developing infections that occur in immunocompetent patients but also for infections that do not affect patients with normal immunity (opportunistic infections). Awareness of the type and severity of the immunologic defect can be helpful in predicting the most likely organisms responsible for the infection. For example, gram-negative bacteria, Aspergillus and Candida, should be the primary considerations in patients with severe neutropenia.
Imaging Approach
Chest radiography has been shown to be important in the diagnosis and management of immunocompromised patients with a suspected respiratory infection (1,23,24,25,26). It remains the first and foremost imaging modality used in the evaluation of these patients and in most cases provides adequate imaging information. However, it has limited sensitivity for the detection of early infection being normal in up to 10% of patients with proved pulmonary disease (27,28). Serial chest radiographs are often requested to detect pulmonary disease, but faint opacities may be difficult to detect, especially in patients who are unable to take a full inspiration. Furthermore, neutrophil counts are often low, resulting in a poor inflammatory response, which may further decrease the sensitivity of the chest radiograph.
Computed tomography (CT) scan is more sensitive and specific than chest radiography in the detection of subtle pulmonary abnormalities. CT scan is particularly helpful in the assessment of patients with acute pulmonary disease and a high clinical suspicion for pneumonia but with normal or questionable radiographic findings. Heussel et al. (26) performed CT scan in neutropenic patients with unexplained fever and a normal chest radiograph. CT scan demonstrated findings consistent with pneumonia in 60% of cases 5 days before any abnormalities were evident on the chest radiograph.
The most common patterns seen on high-resolution CT scan in acute pulmonary infections are nodules,
centrilobular branching linear and nodular opacities (“tree-in-bud” pattern), ground-glass opacities, consolidation, or a combination of these (see Table 8.1) (1,28). Ground-glass opacities are a common but nonspecific CT scan finding that may result from bacterial, fungal, or viral pneumonia (29,30,31) or from noninfectious conditions such as drug-induced lung disease, pulmonary edema, and pulmonary hemorrhage. However, in severely immunocompromised patients, particularly following hematopoietic stem cell or organ transplantation, extensive bilateral ground-glass opacities should raise the possibility of Pneumocystis or cytomegalovirus (CMV) pneumonia.
centrilobular branching linear and nodular opacities (“tree-in-bud” pattern), ground-glass opacities, consolidation, or a combination of these (see Table 8.1) (1,28). Ground-glass opacities are a common but nonspecific CT scan finding that may result from bacterial, fungal, or viral pneumonia (29,30,31) or from noninfectious conditions such as drug-induced lung disease, pulmonary edema, and pulmonary hemorrhage. However, in severely immunocompromised patients, particularly following hematopoietic stem cell or organ transplantation, extensive bilateral ground-glass opacities should raise the possibility of Pneumocystis or cytomegalovirus (CMV) pneumonia.
TABLE 8.1 High-Resolution Computed Tomography Patterns in Pulmonary Infections: Most Common Causes | ||
|---|---|---|
|
In the appropriate clinical setting, high-resolution CT scan findings may result in a change in clinical management or add confidence to the diagnosis. For example, focal airspace consolidation, with or without cavitation, has been shown to be most commonly caused by bacterial infection. Less commonly, it may result from invasive aspergillosis and mycobacterial infection. A predominantly nodular pattern is seen in a variety of infections. The presence of a halo of ground-glass attenuation, indicating hemorrhage surrounding the nodule, is characteristic of angioinvasive aspergillosis (32,33,34). Although the CT scan halo sign in patients with severe neutropenia is most suggestive of invasive aspergillosis, a similar pattern may also be seen in candida, CMV, varicella, and herpes simplex pneumonia (35,36,37).
Transplant Recipients
Pneumonia is a common complication following hematopoietic stem cell and solid organ transplantation (8,14,15,16,24,38). The etiology of pneumonia includes bacteria and opportunistic organisms and can be established from sputum culture, bronchoalveolar lavage (BAL), blood culture, or fine needle aspiration (39,40,41).
Hematopoietic stem cell transplantation is currently the treatment of choice for various hematologic malignancies and severe congenital or acquired disorders of the hematopoietic or immune systems (19,42). It is estimated that >50,000 stem cell transplantations are performed annually worldwide (19,42). Infectious and noninfectious pulmonary complications can occur in 40% to 60% of hematopoietic stem cell transplant recipients, being most frequent in allogeneic transplant recipients (26,43).
Complications following hematopoietic stem cell transplantation have been classified according to the time of presentation into early (pre-engraftment phase) and late (postengraftment phase) depending on whether they occur before or after 100 days following transplantation (see Table 8.2) (19,42,44,45). During the initial post-transplantation period, patients are profoundly neutropenic (absolute neutrophil count <500 cells per μL) and most microbiologically documented pneumonias are caused by fungi or bacteria (46). If neutropenia is prolonged beyond 2 weeks, Aspergillus sp and other opportunistic fungi may cause life-threatening infections (47). While fungi are the most common cause of pulmonary infection in the early pre-engraftment phase (first 30 days post-transplantation), viruses most commonly occur in the postengraftment phase. Conversely, in the late postengraftment phase, from day 100 until the patient regains normal immunity, usually 1 to 2 years later, most infections are caused by bacteria (42,45,46).
Solid organ transplantation has become the treatment of choice for patients with end-stage diseases of the kidney, liver, heart, and lung. Renal transplantation accounts for more than half of all solid organ transplantations performed in the United States, and the liver is the second most commonly transplanted solid organ (25,44).
The type of infection following solid organ transplantation is influenced by the type of transplantation and the
time interval since transplantation (48,49,50). Infections are common following lung transplantation and relatively uncommon following kidney transplantation due to the less rigorous surgical procedure required to implant the allograft and the lower level of immunosuppression required to maintain it (43).
time interval since transplantation (48,49,50). Infections are common following lung transplantation and relatively uncommon following kidney transplantation due to the less rigorous surgical procedure required to implant the allograft and the lower level of immunosuppression required to maintain it (43).
TABLE 8.2 Pulmonary Infections After Hematopoietic Stem Cell Transplantation | |
|---|---|
|
Post-transplantation complications have been classified according to the time following the surgical procedure. The post-transplantation period can be subdivided into postoperative (0 to 30 days), early (31 to 180 days post-transplantation) and late (>6 months post-transplantation) (43). Infections are more frequent and most varied during the first 6 months after transplantation (44). In the postoperative period, nosocomial transmission of respiratory viruses and common gram-positive and gram-negative organisms frequently occurs through contaminated hands of hospital personnel. After the first 6 months, the risk of infection correlates directly with the degree of immunosuppression needed to forestall graft rejection (43).
The differential diagnosis of pulmonary infiltrates in transplant recipients remains a difficult diagnostic challenge (1,7,42). The differential diagnosis is broad and includes both infectious and noninfectious causes such as hemorrhage, drug-induced lung disease, pulmonary edema, and pulmonary embolism (45). Unfortunately, the clinical data and radiographic findings often fail to lead to a definitive diagnosis of pneumonia because there is an extensive number of noninfectious processes associated with fever and pneumonitis that may mimic pulmonary infection, including drug-induced pulmonary disease, organizing pneumonia, and pulmonary vasculitis (1,27,45).
Aspirates obtained during fiberoptic bronchoscopy and BAL have the highest diagnostic yield and impact on therapeutic decisions (51). Diagnostic information may also be obtained by transbronchial biopsy or percutaneous needle aspiration (52,53). The combined use of clinical information, knowledge of typical conditions associated with the host’s immunodeficiency, and radiographic patterns offers a useful approach to the diagnosis of pulmonary disease in these patients.
Fungi
Fungal infections are responsible for >10% of pulmonary infections in hematopoietic stem cell transplant recipients and approximately 5% of pulmonary infections in solid organ transplant recipients (34,54,55). Fungal infection can occur at any time but invasive disease occurs most commonly within the first 6 months of transplantation. The main risk factors for invasive aspergillosis are severe neutropenia and prolonged high-dose corticosteroid therapy (50).
Pneumocystis jiroveci (carinii), Aspergillus fumigatus, and Candida albicans are the most common fungi causing pulmonary infection in immunocompromised patients. Other less common fungal pathogens are Cryptococcus neoformans, Mucor, and endemic fungi such as Histoplasma capsulatum, Coccidioides immitis, and Blastomyces.
Pneumocystis Jiroveci (Carinii)
Pneumocystises are unicellular organisms currently classified as fungi. They include several species that are host-specific. The Pneumocystis that infects humans does not infect animals and was recently renamed P. jiroveci. Pneumocystis carinii on the other hand infects only rats. P. jiroveci causes pneumonia only in immunocompromised patients, particularly patients with AIDS, lymphoproliferative disorders, and with organ or hematopoietic stem cell transplantation (see Table 8.3) (1,12,55,56,57). The organism probably resides normally on the alveolar surface, where it is maintained in low numbers by host defense mechanisms. The most common histologic pattern of pulmonary infection consists of finely vacuolated eosinophilic material within alveolar airspaces accompanied by a variably severe infiltrate of lymphocytes and plasma cells in the adjacent interstitium. The foamy material consists of solitary and encysted organisms, which can be detected with special stains as round or helmet-shaped structures admixed with host-derived material such as surfactant and fibrin. Other histologic reaction patterns include granulomatous inflammation and diffuse alveolar damage (58). Patients typically present with insidious symptoms of fever, nonproductive cough, and dyspnea. A definitive diagnosis of Pneumocystis pneumonia (PCP) requires the demonstration of organisms in sputum or BAL fluid.
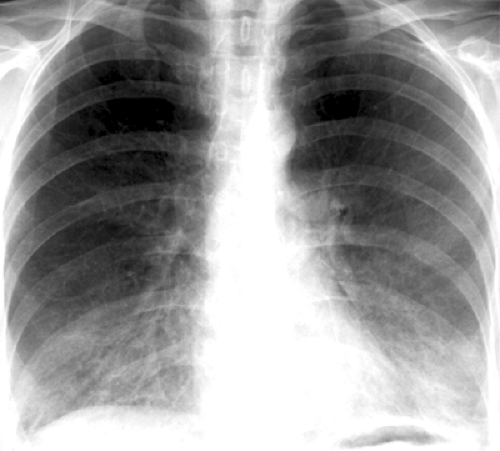
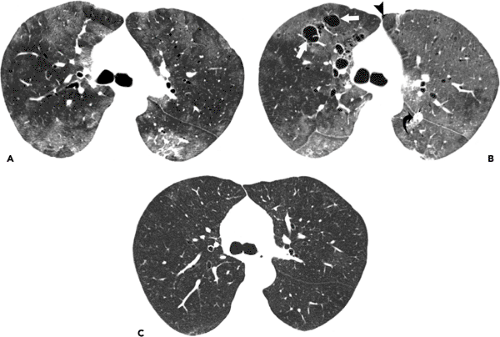
The characteristic initial radiographic manifestation is that of bilateral symmetric ground-glass
opacities. These tend to involve mainly the perihilar regions but may be diffuse or involve mainly the lower or upper lung zones (see Fig. 8.1). Unless the patient is treated, the ground-glass opacities progress over 3 to 5 days to homogeneous diffuse airspace consolidation. The pattern may be mistaken for pulmonary edema, but the heart size is usually normal (59). Hilar lymphadenopathy and pleural effusion are distinctly unusual.
opacities. These tend to involve mainly the perihilar regions but may be diffuse or involve mainly the lower or upper lung zones (see Fig. 8.1). Unless the patient is treated, the ground-glass opacities progress over 3 to 5 days to homogeneous diffuse airspace consolidation. The pattern may be mistaken for pulmonary edema, but the heart size is usually normal (59). Hilar lymphadenopathy and pleural effusion are distinctly unusual.
TABLE 8.3 Pneumocystis Pneumonia | ||
|---|---|---|
|
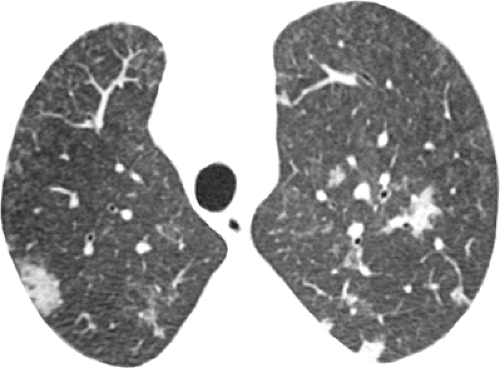
The characteristic high-resolution CT scan manifestations of PCP include extensive symmetric bilateral ground-glass opacities (see Fig. 8.2). Small nodules, foci of consolidation, and linear opacities may be seen in association with the ground-glass opacities (see Figs. 8.3 and 8.4). The presence of septal lines and smooth intralobular linear opacities superimposed on the ground-glass opacities results in a pattern known as crazy paving (60,61,62,63). Sharp demarcation between normal and abnormal parenchyma results in a mosaic or geographic appearance (see Fig. 8.5).
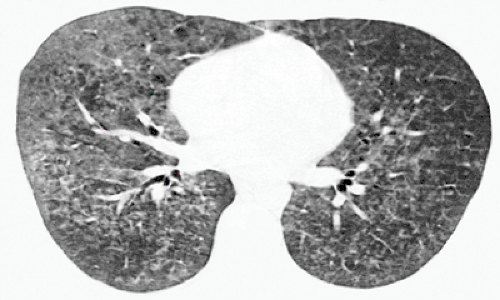
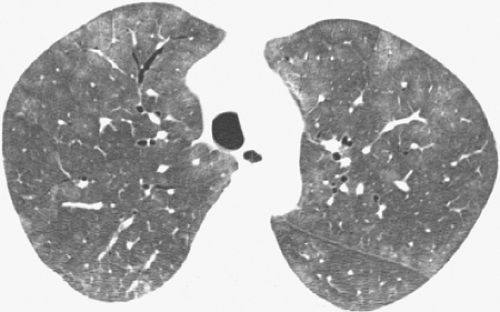 Figure 8.2 Pneumocystis pneumonia (PCP) in lymphoma. High-resolution computed tomography (CT) image (1-mm collimation) at the level of aortic arch shows extensive bilateral ground-glass opacities. The findings are a common and characteristic feature of PCP. The patient was a 55-year-old woman (same patient as in Figure 8.1). |
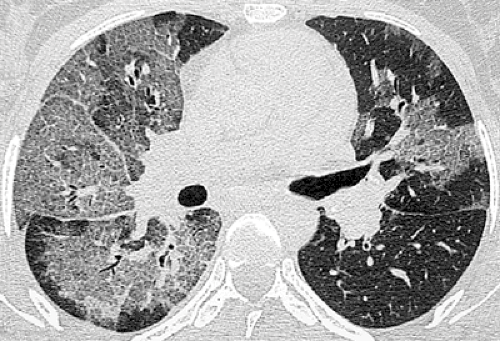
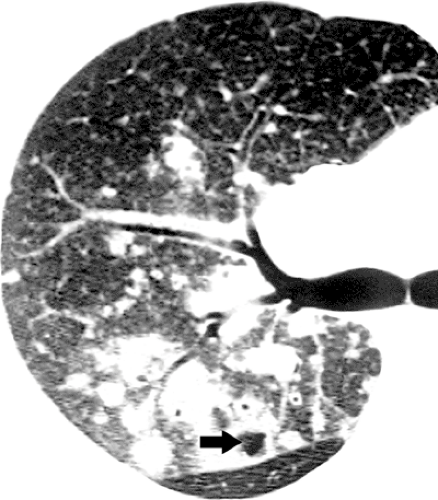
Thin-walled cystic spaces superimposed on the ground-glass opacities are relatively uncommon in non–AIDS patients. The cystic lesions represent pneumatoceles and are associated with an increased prevalence of pneumothorax (64,65). The cysts are usually multiple and tend to decrease in size or resolve after the acute stage of the infection (see Fig. 8.6). They are most common in the upper lobes.
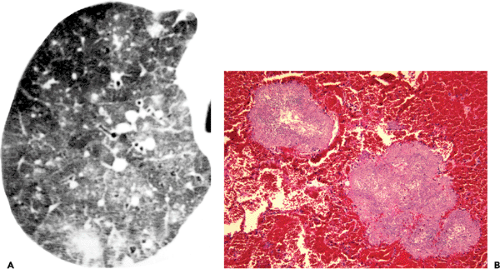
Atypical manifestations of PCP include miliary nodules, nodular areas of consolidation (see Fig. 8.7), large nodules, and focal masses or mass-like areas of consolidation.
Candida Albicans
Candida albicans is a ubiquitous dimorphic fungus identified in tissue as both oval budding yeast and hyphae. Pulmonary candidiasis is seen mainly in patients with
hematologic malignancies (acute leukemia and lymphoma) and in intravenous drug users (see Table 8.4) (49). It usually accompanies widespread infection of the urinary tract, gastrointestinal tract, liver, spleen, or central nervous system. Factors that predispose hematopoietic stem cell transplant recipients to candida infections include allogeneic transplant, increased age, and a prolonged neutropenia (49,66).
hematologic malignancies (acute leukemia and lymphoma) and in intravenous drug users (see Table 8.4) (49). It usually accompanies widespread infection of the urinary tract, gastrointestinal tract, liver, spleen, or central nervous system. Factors that predispose hematopoietic stem cell transplant recipients to candida infections include allogeneic transplant, increased age, and a prolonged neutropenia (49,66).
The chest radiographic manifestations include patchy unilateral or bilateral airspace consolidation and poorly defined nodules (67,68). These findings reflect the presence of necrotizing bronchopneumonia. Occasionally, miliary disease is seen (68).
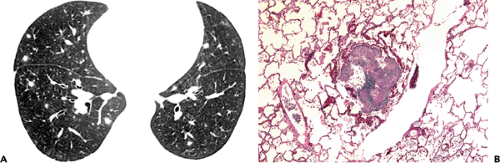
Franquet et al. (69) evaluated the high-resolution CT scan findings in 17 hematopoietic stem cell transplant recipients with histopathologically proved pulmonary candidiasis. Multiple nodules and tree-in-bud opacities were common, being seen in 15 (88%) of 17 patients and 7 (41%), respectively (see Fig. 8.8). The nodules were bilateral in 12 patients and unilateral in 3. Nodules were
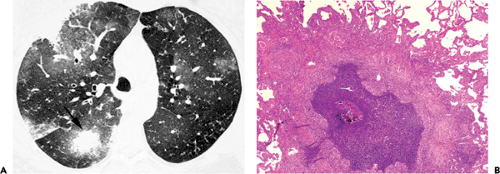
an isolated CT scan finding in 5 patients. An associated halo of ground-glass opacity was present in five of 15 (33%) patients with nodules (see Fig. 8.9). Airspace consolidation was present in 11 (65%) patients and ground-glass opacities in 6 (35%) (see Figs. 8.9 and 8.10). They concluded that the most common high-resolution CT scan findings of pulmonary candidiasis in hematopoietic stem cell transplant recipients are multiple bilateral nodular opacities often associated with consolidation. Definitive diagnosis of pulmonary candidiasis requires demonstration of the organism in tissue (68,69).
an isolated CT scan finding in 5 patients. An associated halo of ground-glass opacity was present in five of 15 (33%) patients with nodules (see Fig. 8.9). Airspace consolidation was present in 11 (65%) patients and ground-glass opacities in 6 (35%) (see Figs. 8.9 and 8.10). They concluded that the most common high-resolution CT scan findings of pulmonary candidiasis in hematopoietic stem cell transplant recipients are multiple bilateral nodular opacities often associated with consolidation. Definitive diagnosis of pulmonary candidiasis requires demonstration of the organism in tissue (68,69).
TABLE 8.4 Pulmonary Candidiasis | ||
|---|---|---|
|
Aspergillus Fumigatus
Pulmonary aspergillosis is usually acquired by inhalation of the organisms normally present in the environment. It occurs almost exclusively in individuals who have structural lung abnormality (such as a cavity), atopy, or deficiency of the inflammatory or immunologic reactions. The pathologic and radiologic manifestations of the disease can be divided into three main forms: Aspergilloma, allergic bronchopulmonary aspergillosis (ABPA), and invasive aspergillosis. The last named in turn can be subdivided into
angioinvasive, bronchopneumonic (airway invasive), and chronic necrotizing (semi-invasive) forms (64,65,70,71,72).
angioinvasive, bronchopneumonic (airway invasive), and chronic necrotizing (semi-invasive) forms (64,65,70,71,72).
Invasive Pulmonary Aspergillosis
Invasive pulmonary aspergillosis occurs only in immunocompromised patients and it is the most common opportunistic pulmonary fungal infection. Risk factors for invasive aspergillosis include severe or prolonged neutropenia (absolute neutrophil count <500 per mm3), prolonged corticosteroid therapy, graft-versus-host disease after hematopoietic stem cell transplantation, and late-stage AIDS (see Table 8.5) (32,50). Infection begins when aerosolized spores are inhaled into the distal airways and airspaces. In the absence of an effective host immune response, the spores mature into hyphae that can invade the pulmonary arteries. This results in pulmonary arterial thrombosis, hemorrhage, lung necrosis, and systemic dissemination (50,73,74).
Affected patients present with fever, cough, and dyspnea. Symptoms suggestive of pulmonary embolism, such as pleuritic chest pain, may also occur. The diagnosis of invasive aspergillosis is difficult because the organism can normally colonize the upper airway. The diagnosis is based on clinical, radiologic, and mycological data. On the basis of the findings, the likelihood of Aspergillus infection can be classified into proved, likely, and possible (10). Specimens obtained from normally sterile but clinically abnormal sites (e.g., needle biopsy of the lung lesion or surgical lung biopsy) are the most reliable and considered necessary to prove the diagnosis. Mycologic evidence acquired by means of either direct examination or culture of specimens from sites that may be colonized (e.g., sputum, BAL fluid) are helpful in supporting the likely diagnosis but do not prove the diagnosis. Similarly, galactomannan and nucleic acid detection in serum or in BAL fluid are supportive of the diagnosis but do not prove it; definite diagnosis of invasive aspergillosis requires the demonstration of the fungus in tissue specimens (75). Furthermore, the potential value of early diagnostic tests such as the galactomannan needs to be confirmed in prospective trials (76).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree