MI
Stroke
Risk ratio (95 % CI)
Risk ratio (95 % CI)
SLE
2.25 (1.37–3.68)
2.29 (0.85–6.15)
RA
2.10 (1.52–2.89)
1.91 (1.73–2.12)
SSc
1.82 (1.40–2.36)
1.43 (1.12–1.83)
Systemic vasculitis
2.50 (1.60–3.70)a
0.74 (0.44, 1.26)b
1.40 (0.60–3.30)c
pSS
2.40 (1.50–3.80)
1.60 (1.00–2.80)
In comparison with the general population, atherosclerosis occurs earlier and progresses more rapidly in immune-mediated inflammatory rheumatic diseases. The chronological age of the patients is not concordant with the state of their arteries, which are ageing prematurely. The mechanisms underlying accelerated atherosclerosis are complex and represent an area of intense investigation. Systemic rheumatic diseases can be a useful model to expand the understanding of mechanisms behind atherogenesis.
Although traditional cardiovascular risk factors predict CV outcomes, they do not fully explain the excessive risk observed in patients suffering from inflammatory rheumatic diseases. Competing risks such as the disease itself and disease-specific factors, including chronic inflammation, vasculopathic changes and also the effect of medication, are among the most widely accepted hypothesis. It has been suggested that despite the overall increased risk, the relative contribution of traditional CV risk factors might be smaller due to the presence of those competing risks [12, 13].
Systemic Inflammation as the Main Driver of Cardiovascular Risk in Rheumatic Diseases
The atherosclerotic process begins early in life with the accumulation of lipid-laden cells beneath the endothelium – the fatty streak – that includes mainly macrophages and some T cells. The fatty streak can vanish or may progress to atheroma formation. Atheromata consist of cells, connective tissue elements, lipids and debris that together thicken the intima. Inflammatory and immune cells constitute an important part of the atherosclerotic lesions. LDL cholesterol infiltrates the wall of large- and medium-sized arteries where it is responsible for initiating an inflammatory response. LDL oxidation evokes the release of phospholipids that activate the endothelium mainly at sites of hemodynamic strain (high oscillatory, but low average shear stress), which leads to increased expression of adhesion molecules and inflammatory cytokines. Blood platelets adhere to surface molecules of endothelial cells via their glycoproteins IIb/IIIa.
The atheroma possesses a central core with foam cells and extracellular lipid droplets surrounded by a cap of smooth muscle cells and a collagen-rich matrix. Atheroma growing involves infiltration by T cells, macrophages and mast cells; many of them are activated and produce pro-inflammatory cytokines (interleukin-1β, tumour necrosis factor, γ-interferon) that promote the migration and proliferation of smooth muscle cells and the construction of a dense extracellular matrix – the advance atherosclerotic lesion.
The acute coronary syndromes are usually initiated by a rupture of the fibrous cap but in some cases can occur just by endothelial erosion. In one quarter of cases, the endothelium does not rupture, but instead it is replaced for prothrombotic inflammatory cells. Plaque rupture usually occurs in areas of sustained inflammation, macrophage accumulation and apoptosis. Proteolytic enzymes produced by activated macrophages can degrade the collagen of the fibrous cap facilitating its rupture. These enzymes belong to the matrix metalloproteinase (MMP) family. The three MMPs [1, 8, 13] are overproduced by macrophages present in lesions where massive thrombosis has occurred. Furthermore, it seems to be a crosstalk between this immune effector macrophage pathway and adaptive immune cells (T cells) inhibiting the synthesis and augmenting the degradation of interstitial collagen. In fact, it has been demonstrated that T-cell-derived cytokine CD40 ligand (CD154) boosts the production of interstitial collagenase by human macrophages.
The observations above provide a cellular and molecular mechanism linking inflammation to the thinning and weakening of the fibrous cap, which can precipitate plaque rupture, thrombosis and acute CV events. Longitudinal studies have demonstrated that the overall disease activity, a proxy of inflammatory burden, is crucial for cardiovascular outcome of immune-mediated inflammatory rheumatic diseases [14]. Steiman et al. showed in SLE patients the predictive value of disease activity by demonstrating that clinically active lupus patients are at higher CV risk than serologically active clinically quiescent patients. After a 10-year follow-up, 7.3 % of clinically active patients developed new coronary events comparing to 1.8 % of those with clinically quiescent disease [15]. Likewise, in RA patients, systemic inflammation and CV risk factors predict greater IMT progression rate [16]. High levels of inflammatory cytokines generate a spectrum of pro-atherogenic changes that fosters atherosclerosis development and progression. A direct effect on the vasculature can be observed throughout the whole process, that is, from early endothelial dysfunction to plaque rupture and the occurrence of a thrombotic event (Fig. 6.1). Not only endothelial function and repair are negatively affected by increased circulating levels of pro-inflammatory cytokines such as tumour necrosis factor (TNF), interleukin (IL)-Iβ or IL-6 but also high levels of inflammation are associated with plaque instability and rupture.
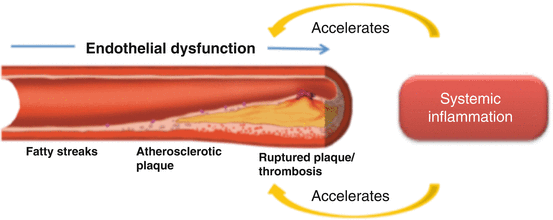

Fig. 6.1
The interplay of systemic inflammation and atherosclerosis progression
In addition, systemic inflammation affects several other pathways through which may contribute to accelerated atherogenesis:
Insulin resistance
Dyslipidaemia
Prothrombotic status
Oxidative stress
Abnormal body composition phenotype
The association between inflammatory biomarkers and the risk for future CV events has been recognized in several clinical and epidemiological observations. While high levels of C-reactive protein (CRP) are present in major inflammatory situations, high-sensitivity (hs) CRP elevation coexists with states of low-grade inflammation such as atherosclerosis. CRP has been associated with a number of CV risk factors like obesity, smoking, heart rate, high blood pressure, high serum fibrinogen, hypercholesterolaemia, hypertriglyceridaemia, apolipoprotein B or increased fasting blood glucose. In the general population, hsCRP is an independent predictor of future vascular events at any level of LDL cholesterol and, in association with LDL cholesterol levels, it improves the individual estimation of CV risk.
Nonetheless, in patients with rheumatic diseases, where highly increased CRP is a characteristic, the relationship of this inflammatory biomarker to subsequent clinical events has not been consistently established.
Endothelial Activation and Dysfunction
Endothelial cells (EC) are crucial in maintaining blood fluidity and in regulating vascular tonus and permeability. Under normal conditions, EC express molecules that prevent platelet aggregation and clot formation. Inflammatory cytokines modify endothelial homeostasis that results in increased expression of adhesion molecules, loss of antithrombotic properties, increased permeability and reduced ability to produce NO in response to several stimuli. Intercellular adhesion molecules and chemokines mediate leukocyte adhesion to endothelial cells or extracellular matrix, endothelial transmigration and leukocyte activation. Endothelial activation and dysfunction are chief phenomena in early atherogenesis and precedes structural vascular changes. Endothelial dysfunction is potentially reversible with the control of the underlying inflammatory condition.
Along with non-invasive endothelial function testing (e.g. endothelium-dependent flow-mediated dilation, peripheral artery tonometry, pulse wave velocity measurement), circulating biomarkers of endothelial activation have been studied in systemic rheumatic diseases. Increased levels of E-selectin, sICAM-1, VCAM, thrombomodulin (TM) and tissue factor (TF) were identified in relation to disease activity [17].
Another surrogate marker of EC function is the number of circulating endothelial progenitor cells (EPC) and endothelial microparticles. EPC are a heterogeneous cell population derived from the bone marrow involved in angiogenesis and repair of endothelium. Variations in the level of circulating and mature EPC or EPC impaired function have been documented in active RA, SLE and pSS, suggesting compromised endothelial repair [18, 19].
Inflammation and Atherothrombosis
The progression of atherosclerosis and local thrombosis is closely linked phenomena that may culminate in a thrombotic occlusion of the vascular lumen and consequent acute CV event. Alterations of haemostatic and haemorheologic factors contribute to atherogenesis and are related to vascular ischemic events in the general population [20].
In immune-mediated inflammatory rheumatic diseases, both altered blood rheology and high levels of haemostatic biomarkers have been found in association with subclinical atherosclerosis [21]. Serum levels of haemostatic factors, namely, von Willebrand factor (vWF), plasminogen activator inhibitor (PAI)-1, thrombomodulin and tissue factor, are related to inflammatory status. Some of these markers (PAI-1 and vWF) were studied in RA patients and baseline values found to be independent predictors of future CV events.
Patients have reduced erythrocyte deformability and increased erythrocyte aggregation, which may contribute to impaired microcirculation and microvascular dysfunction. The interaction between inflammation and thrombogenesis is not entirely understood. Nevertheless, we and others found disturbed and unfavourable haemorheologic phenotype in association with active RA and active SLE, and this might represent an additional link between inflammation and atherogenesis [21].
Autoantibodies
The contribution of autoantibodies to atherogenesis remains unclear in the scope of rheumatic diseases, yet the current evidence suggests a relationship between some autoantibodies and subclinical atherosclerosis. Increased production of anti-endothelial cell antibodies, a putative marker of vascular damage, has been reported in SLE, RA, systemic vasculitis and other rheumatic diseases [22]. Oxidation is one of the major aspects involved in atheroma development, and high levels of antibodies against oxidized low-density lipoproteins (ox-LDL), a quantifiable marker of oxidative stress present over a certain time period, are found in a significant proportion of lupus patients [23], although their biological role is not well established.
Antiphospholipid antibodies, a family of autoantibodies directed against phospholipid-binding plasma proteins, are strongly associated with thrombosis and pregnancy morbidity. Anti-cardiolipin (aCl) and anti-β2 glycoprotein I antibodies are the most extensively studied, but other antiphospholipid (aPL) antibodies are implicated in CV events through thrombotic pathways. It has been hypothesized that non-thrombotic mechanisms might also relate aPL and CV events. A possible mechanism linking aPL and atherogenesis is their role in inducing oxidative stress. Moreover, anti-cardiolipin antibodies directly interfere with paraoxonase activity, a high-density lipoprotein-related antioxidant enzyme, adding to the oxidative stress found in these conditions [24]. In fact, some small studies found an association between aPL titres and features of subclinical atherosclerosis (increased IMT and CAC score). Furthermore, low levels of IgM anti-oxidized cardiolipin and anti-oxidized phosphatidylserine were found more frequently in lupus patients with carotid plaques compared to those without plaques [25]. More recently, the presence of aPL was shown to be predictive of CAC score > 0 at 15 and 20 years in the large Coronary Artery Risk Development in Young Adults (CARDIA) cohort [26].
Control of Inflammation and CV Risk
Evidence shows that treatment of inflammation reduces CV burden related to systemic rheumatic diseases. The effective control of disease activity can revert early vascular changes measured by endothelium-dependent flow-mediated dilation (FMD), prevent progression of subclinical atherosclerosis and more importantly reduce incident CV events and CV death. Methotrexate is a disease-modifier antirheumatic drug (DMARD) broadly used for the treatment of chronic inflammatory rheumatic disorders. There is substantial documentation that its use not only controls arthritis and improves disease-specific outcomes but significantly reduces the risk of CV events and death in RA patients [27]. A recent meta-analysis reports a 21 % lower risk for fatal and nonfatal hard CV events in methotrexate users (RA, psoriasis and polyarthritis) after a median follow-up of 5.84 years (relative risk 0.79; 95 % CI 0.73–0.87), and risk reduction was even greater when analysis was adjusted for disease activity [28]. More recently, the introduction of biological therapies revolutionized the control of clinical manifestations and of structural damage progression in an important proportion of patients who failed synthetic DMARDs. Inhibition of TNF, available for the last 15 years, is associated with improvement of endothelial function [29], prevention of atherosclerosis progression [30] and reduction of risk for all cardiovascular events [31]. The impact of biologics with other mode of action remains largely unknown, although improvement of endothelial function was documented in the short term after IL-6 blockade.
Other antirheumatic medications may also have a beneficial effect on atherogenesis, but except for antimalarials, data is limited. The use of hydroxychloroquine is associated with reduced risk of subclinical atherosclerosis, CV events and overall mortality in lupus patients, but the mechanisms underlying this benefit remain largely undetermined.
The debate around the effect of corticosteroids in atherogenesis continues. If on the one hand corticosteroids have a potent anti-inflammatory effect, on the other hand, this group of drugs has a negative impact on CV risk factors, including weight gain, worsening insulin resistance, hypertension and hyperlipidaemia. Thus, the balance between risk and benefit depends on corticosteroid dosage and duration of treatment.
The Examples of Systemic Rheumatic Diseases
Rheumatoid Arthritis
Rheumatoid arthritis (RA) is the prototype of chronic inflammatory arthropathy that affects 0.5–1 % of the adult world population. Several studies, mainly from Europe, have clearly demonstrated that patients with RA have reduced life expectancy largely as a consequence of an excess of CV deaths. In a recent review of CV comorbidity in rheumatic diseases, Symmons et al. report the results of a meta-analysis of 24 mortality studies in RA published between 1970 and 2005 showing a weighted combined all-cause standardized mortality ratio (met-SMR) of 1.50 (95 % 1.39–1.61) with similar increases for IHD (met-SMR 1.59; 95 %CI 1.46–1.73) and stroke (met-SMR 1.52; 95 % CI 1.40–1.67) [13]. The excess of CV deaths is not apparent at disease onset; it is rather a feature of established RA, positive for rheumatoid factor. Besides fatal events, the risk of nonfatal myocardial infarction (MI) and stroke is about two times higher in RA compared to sex- and age-matched controls [6]. Similarly, subclinical atherosclerosis is identified more frequently in rheumatoid arthritis. Carotid ultrasound is an easy to perform non-invasive method to assess the presence of plaques and the intima-media thickness (IMT). Carotid and coronary atheroscleroses are highly correlated and independent predictors of CV events. A significantly higher prevalence of carotid plaques was shown in RA in comparison with sex-, age- and ethnicity-matched controls (44 % vs 15 %; p < 0.001) [32].
Concerning carotid IMT, results are controversial, and not all studies have established an association between greater IMT in RA diagnosis [32]. IMT correlates with the presence of traditional CV risk factors, but it seems to be a less robust predictor of CV events. Coronary artery calcium (CAC) is another surrogate marker of atherosclerosis that helps CV risk stratification in the general population. The prevalence and the extent of CAC is larger in RA (47.6 %) than in controls (35.2 %) [33]. Macrovascular and microvascular functions are frequently impaired in parallel with disease activity. Using brachial artery FMD, many groups have shown impaired endothelial function in RA. Endothelial dysfunction is related to disease activity and reverts with the treatment of active RA.
Traditional CV Risk Factors
The burden of traditional CV risk factors is high in RA. Hypertension affects more than 40 % of patients, and although its overall prevalence is similar in RA and controls, evidence suggests it may be higher in younger patients and linked to active disease. The inflammatory process affects not only lipid concentration but also function. Dyslipidemia is common and correlates with disease activity. Lipid alterations, mainly reduced HDL cholesterol and increased triglycerides, are present in about half of the patients and may precede the diagnosis of RA. Inflammation alters HDL structure, and HDL loses its anti-inflammatory, antioxidant and cardioprotective properties and may become pro-inflammatory (piHDL). Also insulin resistance and diabetes have been consistently reported more frequently in RA.
Nontraditional Biomarkers
Biomarkers of endothelial activation (VCAM-1 and ICAM-1) are elevated in RA patients and correlate with subclinical atherosclerosis. IL-6 is more strongly associated with endothelial dysfunction than CRP or ESR.
In line with longitudinal studies in the general population, haemostatic factors PAI-1 and vWF were found to be strongly predictive of new CV events in established RA. Also serum levels of asymmetric dimethylarginine (ADMA) are elevated in untreated early arthritis and have a negative effect in coronary flow reserve. After treatment of arthritis, ADMA concentration decreases.
As expected, RA patients have higher levels of inflammatory biomarkers than controls. Some studies, but not all, found increased levels of CRP, IL-1, IL-6 and TNF to be associated with impaired FMD and increased IMT [34].
Many other surrogate markers of endothelial activation, including MCP-1, adiponectin, have been studied in RA as potential biomarkers of atherosclerosis. Nevertheless, the cross-sectional design of most studies, the limited number of participants and the heterogeneity of the endpoints hamper definitive conclusions regarding the reported associations.
RA Medication
As stated before, the control of inflammation has documented benefit on atherogenesis. The use of methotrexate and of biologics decreases CV risk. The benefit was demonstrated not only for subclinical disease but also most importantly the reduction of CV events and also mortality was recognized. On the other side, the use of NSAIDs and corticosteroids aggravates some traditional CV risk factors.
Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) is a multiorgan disease characterized by the production of autoantibodies with an estimated prevalence of 70–110/100,000 inhabitants in South European countries. SLE affects preferentially women of reproductive age and has a mortality risk of over three times that of the population. The higher proportion of deaths beyond 5 years of diagnosis is attributable to atherosclerosis. The prevalence of premature atherosclerosis is remarkable in young females. Most studies report a two- to tenfold increase in the risk of MI among SLE patients, although the relative risk may be as high as 52-fold in women with lupus aged 35–44 years [35]. SLE patients have also increase risk of congestive heart failure and stoke. Again, the relative risk of cerebrovascular disease is higher among premenopausal women. Many studies addressed subclinical atherosclerosis and consistently documented higher prevalence of carotid plaques and CAC score. Regarding cIMT, it is not clear whether it is increased or not. While some groups found greater cIMT, others found no difference between lupus patients and controls.
Endothelial dysfunction, assessed by FMD, is an early event frequently reported among patients with SLE.
Substantial amount of research has focused on the identification of risk factors, and similar to RA, traditional CV risk factors and disease-related and treatment-related factors all have been associated with premature atherosclerosis in lupus.
Traditional cardiovascular risk factors are highly prevalent in SLE. Hypertension was reported in 33 % of SLE patients from the Toronto Lupus Cohort compared to 13 % of age-matched controls. Hypertension is associated with a one- to twofold increased risk of coronary artery disease. Hypercholesterolemia is common and likewise associated with increased risk for CV events. Older age and male gender are also predictors of CV events in lupus. The risk for CV events is four times higher in male patients compared to females.
Nontraditional Biomarkers
Contrasting to the general population where hsCRP is a predictor of subsequent CV events and reflects subclinical inflammation, this association is less consistent in lupus patients. Nevertheless, in the LUMINA cohort, elevated CRP was associated with increased CV risk, and another study also found high hsCRP (above 1.6 mg/l) associated with a HR 3.37 for MI and angina.
Pro-inflammatory HDL (piHDL) can be detected in up to 45 % of SLE patients and correlates with cIMT and carotid plaques.
Some autoantibodies have been considered as possible predictors of atherosclerosis. Antiphospholipid antibodies are found in approximately one-third of lupus patients. The positivity for aPL presents a four-time higher risk of having MI, stroke or peripheral vascular disease. Along with increased risk for both arterial and venous thrombosis, there is some evidence linking aPL and carotid IMT. aPLs were described as stimulators of tissue factor (TF) expression on the surface of peripheral blood mononuclear cells, and given the role of TF in atherothrombosis, it was postulated that TF may play a role in lupus CV disease.
A recent study found that defensins are elevated in lupus patients and are predictive of future CV events and subclinical atherosclerosis.
SLE disease itself emerged as an important CV risk, and higher SLE disease activity measured by the SLEDAI was predictive of future CV events in some cohort studies.
SLE Medication
Corticosteroids remain the cornerstone treatment for severe lupus. Their side effects are well established and include the worsening of some traditional CV risk factors. Nevertheless, by controlling disease activity may counteract some negative effects on the vascular system. The effect of corticosteroids on CV disease remains controversial, and probably the relationship is not linear.
On the contrary, the vasculoprotective effect of antimalarials is now well recognized.
Immunosuppressants such as mycophenolate mofetil and cyclophosphamide are likely to reduce CV burden.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


