Figure 36–1.
Typical example of idiopathic VF. This 54 year old female was referred for neurological consultation because of “recurrent seizures”. Her baseline electrocardiogram shows sinus rhythm with normal PR, QRS and QT intervals (panel a). However there are several ventricular extrasystoles with varying coupling intervals, including short-coupled extrasystoles falling on the peak of the preceding T wave (*). Because of the short-coupled extrasystoles the patient was admitted to the Cardiology Department (instead of the Neurology Department). Soon thereafter a self terminating episode of rapid polymorphic ventricular tachycardia was recorded during one of her “seizures” (panel b). Ventricular fibrillation requiring defibrillation was also recorded shortly thereafter (panel c). The patient was diagnosed as “idiopathic ventricular fibrillation” and has been free of ventricular arrhythmias during treatment with quinidine for more than 9 years
Electrocardiogram
The electrocardiogram (ECG) during sinus rhythm has been conventionally defined as normal. It should be noted, however, that when the entity “idiopathic VF” was first defined, the ECG was considered “normal” when pathologies known at the time were excluded [3]. Specifically, the QT was considered normal because it was not prolonged [3]. However, as discussed above, a considerable proportion of patients with idiopathic VF have “relatively short” QT intervals at baseline [18], QT intervals that appear normal but are shorter than those of comparable healthy controls [19, 32], or QT intervals that are normal at baseline but fail to prolong adequately during braydcardia [26, 27]. The T-peak to T-end interval (the interval from the summit to the end of the T wave), which is a marker of the dispersion of repolarization and arrhythmic risk in the long QT syndrome [46] and Brugada syndrome [47], is normal in idiopathic VF [18].
The early repolarization pattern is also observed in idiopathic VF patients far more frequently than among comparable healthy controls [19, 21, 48, 49] and some use the term “early repolarization syndrome” to describe patients with otherwise idiopathic VF who have this ECG pattern [19, 49]. In its most characteristic form, early repolarization in idiopathic VF patients takes the form of a distinct J-wave followed by an horizontal ST-segment [50]. Based on our case control series [21] and using conditional-probability formulas [21] we estimate that (1) the estimated odds for developing idiopathic VF for an individual in the 35–45 years age-range are 3.4 in 100,000; (2) the risk increases to 11 in 100,000 once J-waves are found in the ECG and (3) the risk increases to 30 in 100,000 if the J-waves are followed by horizontal ST segment [50].
Ventricular extrasystoles only rarely occur in patients with idiopathic VF, but when they do, they have varying coupling intervals with some extrasystoles closely coupled to the preceding complex (mean coupling interval = 302 ± 52 ms in our series [5], 297 ± 41 ms in the series of Haissaguerre [35], 300 ± 35 ms in the series of Champagne [51] and ≤340 ms in the series by Nam [49]). Because of the short coupling interval, the extrasystoles fall on the summit or the descending limb of the T wave (Figs. 36.1 and 36.2). In our series [5], all VF episodes started by ventricular extrasystoles falling within 40 ms (with the vast majority falling within 20 ms) of the peak of the T wave. There appears to be an inverse relationship between the coupling interval of the extrasystoles and the risk for malignant arrhythmias with longer bursts of polymorphic VT triggered by extrasystoles with shorter coupling intervals [35]. Nam reported that the arrhythmias in idiopathic VF with early repolarization are preceded by long-short cycles more often than VF in Brugada syndrome [49]. However, in our experience and that of others, arrhythmias in idiopathic VF are (as a rule) not pause dependent [3, 19, 33, 44, 51].
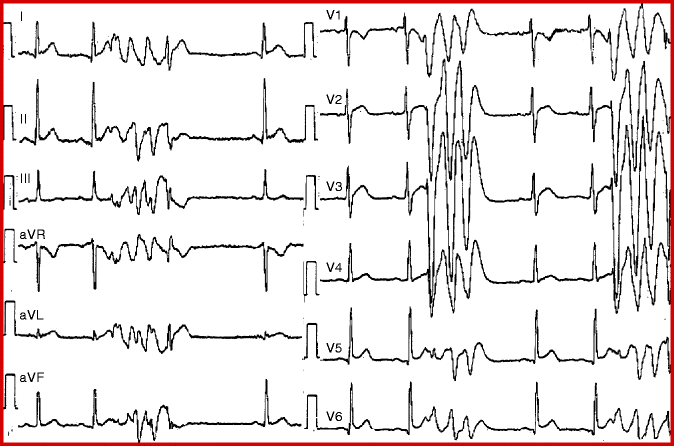

Figure 36–2.
Typical mode of onset of idiopathic VF. Note the very short coupling interval of the extrasystoles initiating the polymorphic ventricular tachycardia. Also, note that despite the polymorphic morphology of the arrhythmia, when more than one VT episode is recorded (like in the precordial leads), the first, second and third complexes of the tachycardias are remarkably similar
Morphology of Extrasystoles
In early reports showing the onset of idiopathic VF [3, 33, 52], a similar ECG pattern of the first short-coupled ventricular extrasystole was observed, namely left bundle branch block pattern and left axis (Fig. 36.2). However, later reports [34, 35], showed that extrasystoles with other patterns do exist, suggesting various possible origin sites. Interestingly, when multiple episodes of polymorphic VT are recorded with 12-lead recordings, the morphology of the initiating beats of all these episodes is similar. This applies not only to the first complex, but to the second and third complexes of the polymorphic arrhythmias as well (Fig. 36.2). The last observation supports the notion that idiopathic VF has a focal origin (see below) [34, 35].
Electrophysiologic Data
Patients with idiopathic VF have normal A-H and H-V intervals, and their ventricular refractory periods are within normal limits [2, 53]. This is in contrast to patients with Brugada syndrome, who often have prolonged H-V interval [12] and patients with short QT syndrome, who have short refractory periods in the atrium and the ventricle [23, 54].
The ventricular arrhythmias induced by programmed ventricular stimulation are invariably of polymorphic morphology, namely polymorphic VT or VF (Fig. 36.3). Induction of monomorphic VT excludes the diagnosis of idiopathic VF. This is at variance with patients with Brugada syndrome who also have primarily VF [55], but rarely have monomorphic VT [56–60].
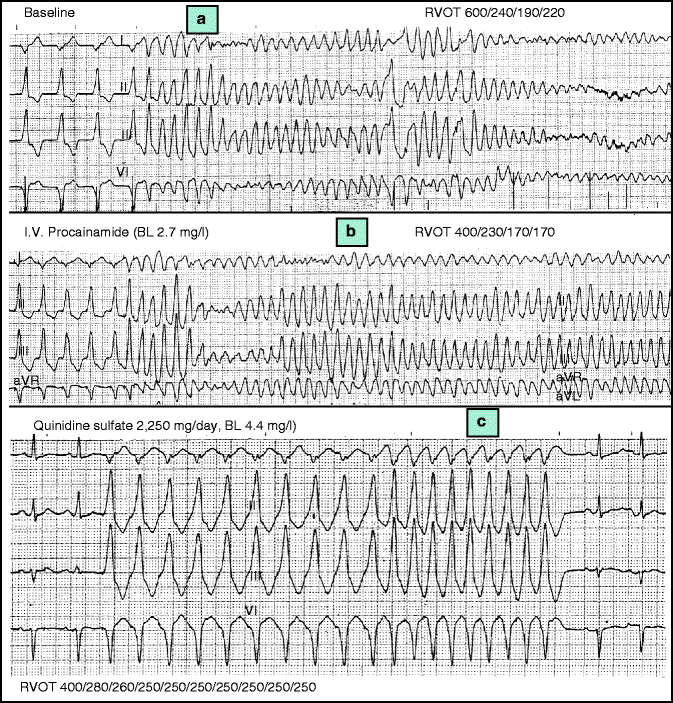

Figure 36–3.
Typical results of electrophysiologic study in idiopathic VF. Panel a: At the baseline study, VF is induced by triple ventricular extrastimulation with short coupling intervals from the right ventricular outflow tract. Basic ventricular pacing at 100 beats/min (cycle length 600 ms) is followed by three extrastimuli 240, 190 and 220 ms apart and this initiates VF that required DC shock for termination. Panel b: After intravenous administration of 1,000 mg procainamide the protocol of extrastimulation is repeated and VF is induced again. Note that despite of therapeutic levels of procainamide the effective refractory period is sufficiently short to allow ventricular capture of the ventricle with short coupling intervals (230, 170 and 170 ms for the first, second and third extrastimuli, respectively). Panel c. After 5 days of oral therapy with quinidine it is no longer possible to capture the ventricle with short coupling intervals. No ventricular arrhythmias could be induced despite a maximally aggressive protocol of extrastimulation, including nine extrastimuli. This cardiac arrest survivor has been free of arrhythmias for >5 years on quinidine therapy
The inducibility rate is a function of the protocol used during programmed ventricular stimulation. Many electrophysiologists are reluctant to shorten the coupling interval of the delivered ventricular extrastimuli beyond a “nominal” value of 200 ms. This is because the risk of “accidentally” inducing VF in healthy individuals also increases as the coupling interval of the second and third extrastimuli are shortened below 200 ms [61–63]. Indeed, in small studies performed 20 years ago, 9 % of healthy individuals – without documented or suspected spontaneous ventricular arrhythmias – had inducible VF when the coupling intervals were limited only by ventricular refractoriness [61–63]. Moreover, an additional 40 % of the last group of healthy controls had inducible non-sustained polymorphic VT and this lead to premature discontinuation of the pacing protocol [61–63]. Therefore, one must recognize that at least 9 % of healthy individuals will have inducible VF if aggressive protocols of extrastimulation (with double and triple extrastimulation with coupling intervals shorter than 200 ms) are used [64]. On the other hand, in our experience, as many as 80 % of patients with idiopathic VF have inducible VF with aggressive protocols of extrastimulation consisting of double and triple ventricular extrastimulation at two right ventricular pacing sites and using repetition of extrastimulation at the shortest coupling interval that captures the ventricle [2, 52, 53]. This very high-inducibility rate suggests that the induction of VF, with aggressive protocols of extrastimulation, is a valid endpoint of programmed ventricular stimulation that then may be used for guiding antiarrhythmic therapy with quinidine in patients with idiopathic VF (Fig. 36.3) (see section “Prognosis and Therapy of Idiopathic VF”).
Recently, Haissaguerre et al. performed endocardial recordings in patients with idiopathic VF at a time when they had frequent spontaneous ventricular extrasystoles and/or bursts of polymorphic VT [34, 35]. The investigators were able to locate the site of origin of these ventricular arrhythmias in 27 patients. Successful localization of the site of origin of the ventricular arrhythmias was guided by recording of very early endocardial recordings and confirmed by abolition of ventricular arrhythmias following radiofrequency ablation of the firing focus. Purkinje potentials were recorded at the site of origin of ventricular arrhythmias in 23 (85 %) out these 27 patients (in the left ventricular septum in ten patients, the anterior right ventricle in nine patients and in both locations in four). The Purkinje potentials preceded the local myocardial activation by 11 ± 5 s during sinus rhythm and by 10 ± 150 ms during spontaneous ventricular ectopy [34, 35]. Based on these endocardial recordings, it seems that the arrhythmias in idiopathic VF have a focal origin and that the triggering focus is within the Purkinje fibers in the majority of patients. Of note, the firing focus was not within the Purkinje network in only four (15 %) patients and in all these patients the arrhythmias originated in the right ventricular outflow tract (RVOT). It is possible that idiopathic VF originating from the RVOT and the “short-coupled variant of RVOT” described by our group [65], and the “malignant idiopathic RVOT-VT” described by Noda [66] represent the same entity.
Diagnosis
Diagnosing idiopathic VF in a cardiac arrest survivor is straightforward when the onset of spontaneous polymorphic VT/VF is recorded (usually during an arrhythmic storm) and this shows initiation of polymorphic VT/VF by very short coupled ventricular extrasystoles (Figs. 36.1 and 36.2) [3, 5]. This is because the only three other conditions that lead to such characteristic mode of VF initiation (myocardial ischemia [67, 68], Brugada syndrome [69] and short QT syndrome [117]) can be identified with appropriate testing.
More often, however, patients are admitted after resuscitation from cardiac arrest and have documented ventricular fibrillation, but recordings of the arrhythmia onset are not available. In such cases, the diagnosis of idiopathic VF is established by excluding all identifiable causes and further supported by the inducibility of VF with programmed ventricular stimulation. A discussion of all potential causes of sudden death is beyond the scope of this chapter, but a practical approach is presented in Fig. 36.4.
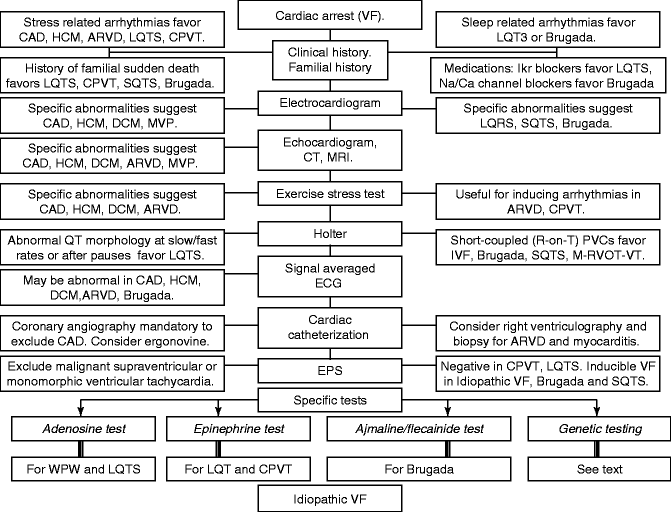

Figure 36–4.
Proposed basic algorithm to diagnose idiopathic VF. Abbreviations in the algorithm are as follows: CAD coronary artery disease, HCM and DCM hypertrophic and dilated cardiomyopathy, respectively, ARVD arrhythmogenic right ventricular dysplasia/cardiomyopathy, LQTS long QT syndrome, CPVT catecholaminergic polymorphic ventricular tachycardia, LQT3 long QT syndrome of LQT3 genotype, SQTS short QT syndrome, MVP mitral valve prolapse, CT computerized tomography, MRI magnetic resonance imaging, M-RVOT-VT malignant form of idiopathic ventricular tachycardia originating from the right ventricular outflow tract, WPW Wolff-Parkinson-White syndrome
The diagnosis of idiopathic VF should be considered in patients presenting with syncope without documented arrhythmias. Having said that, it must be emphasized that in the overwhelming majority of patients presenting with syncope in the absence of heart disease, a diagnosis of vasovagal syncope (rather than arrhythmic syncope) will be evident already from the clinical history. Also, the vast majority of patients with syncope that does not appear to be of vasovagal origin also have electrocardiographic or echocardiographic abnormalities that will suggest an underlying diagnosis. Therefore, only very rarely one is confronted by a patient presenting with syncope in whom the history is sufficiently worrisome to suggest an arrhythmic origin, yet all non-invasive studies, including exercise [70–72] and standing tests [73] to exclude exercise-induced arrhythmias and long QT syndrome, as well as drug challenges to exclude Brugada syndrome [74, 75] long QT syndrome [76, 77] and Wolff Parkinson White [78], are all negative. In such cases, electrophysiologic evaluation can be performed to exclude intra-His block as the cause of syncope. However, recommending the performance of programmed ventricular stimulation to a patient with syncope who has no evidence of heart disease and no documented arrhythmias (particularly closed-coupled ventricular extrasystoles) is problematic. This is because, in the absence of organic heart disease, inducible ventricular arrhythmias (if any), are likely to be of polymorphic morphology. Understanding the significance of inducible VF in the absence of documented spontaneous arrhythmias is difficult because such arrhythmias may be induced in at least 9 % of healthy individuals [64]. Therefore, performance of programmed ventricular stimulation should only be performed when both physician and patient are prepared to accept the induction of VF as a “positive” response.
Differential Diagnosis
Subtle Forms of Organic Heart Disease
Excluding all forms of organic heart disease is essential before the diagnosis of idiopathic VF is considered. However, it should be noted that some forms of organic heart disease may cause malignant ventricular arrhythmias at a time when the anatomic abnormalities are minimal and difficult to detect by imaging modalities. For example, patients with hypertrophic cardiomyopathy due to Troponin-T mutations may be at risk for arrhythmic death at a time when left ventricular hypertrophy is still mild [79, 80]. Similarly, right ventricular dysplasia is sometimes identified as the underlying cause of sudden death only during forensic examination and despite negative extensive diagnostic workup [81]. Of note, subtle anatomic abnormalities, like mitral valve prolapse without hemodynamic significance, should not necessarily be accepted as the cause of cardiac arrest. On the other hand, signs of severe left ventricular dysfunction after resuscitation should not necessarily be used to exclude the diagnosis of idiopathic VF because prolonged resuscitation may result in transient electrocardiographic and echocardiographic abnormalities that are indistinguishable from those seen in patients with dilated cardiomyopathy [82, 83]. If such abnormalities resolve, the diagnosis of idiopathic VF should obviously be considered.
Wolff-Parkinson-White Syndrome
Patients with atrioventricular accessory pathways may have minimal or no ventricular preexcitation (i.e., may have narrow QRS complexes) if they also have fast conduction along the AV node or if their accessory pathways is located on the left lateral wall (far away from the sinus node). Yet, these pathways may have short refractory periods. Such patients may develop atrial fibrillation with rapid ventricular rates that may deteriorate to VF. If the preexcited atrial fibrillation is not recorded and the patient is found in VF, the near-normal electrocardiogram during sinus rhythm may lead to a wrong diagnosis of “idiopathic VF” because all imaging tests will be normal. The wrong diagnosis of idiopathic VF may gain further support from electrophysiologic studies if atrial stimulation is not performed prior to ventricular pacing because programmed ventricular stimulation is likely to induce VF in patients with WPW [84]. Therefore, excluding accessory pathways – either with adenosine injection as a bedside test or with atrial pacing during electrophysiologic studies – is a mandatory step in the work-up of VF survivors even when the electrocardiogram is judged to be “normal”. Of note, rare cases of cardiac arrest caused by very rapid supraventricular tachyarrhythmias in patients without Wolff-Parkinson-White have also been described [85].
Catecholamine Sensitive Polymorphic VT
Physicians may opt to skip performance of exercise testing in cardiac arrest survivors reasoning that coronary angiography will eventually reveal any significant coronary lesion. We have encountered patients with CPVT who were erroneously diagnosed as “idiopathic VF” only because exercise stress testing was not performed. Since all other tests, including electrophysiologic studies, are invariably normal in this disease, exercise stress test is a mandatory test in cardiac arrest survivors. Although the majority of patients with CPVT have a pathognomonic response to exercise (exercise-induced atrial fibrillation followed my multifocal ventricular extrasystoles, bidirectional VT and polymorphic VT), it was recently recognized that some patients with genetically proven CPVT have only single ventricular extrasystoles that look like typical “benign right ventricular outflow tract (RVOT) extrasystoles” during maximal exercise [86, 87]. More recently, a family with CPVT in which affected individuals had negative exercise stress tests but were identifiable by marked post-pause QT prolongation, was described [88]. Such patients could easily be misdiagnosed as “idiopathic VF.”
Long QT Syndrome (LQTS)
The QTc intervals of the healthy population, as well as the QTc of patients with LQTS have a normal distribution and there is considerable overlapping between the QTc of both populations. Importantly, 12 % of patients with genetically proven LQTS have “normal” QT when the latter is defined as QTc <440 ms [89]. Identifying patients with LQTS who have borderline QT is especially challenging in the LQT1 genotype because the T-wave morphology, which is frequently abnormal in LQT2 and LQT3, is most often normal in LQT1. Fortunately, the epinephrine challenge test is especially effective for unraveling abnormal QT responses in LQT1 [76, 77]. We recently described that the sudden tachycardia induced by standing unmasks LQTS in borderline cases and use that as a bedside test [73].
Short QT Syndrome (SQTS)
The newly described SQTS [15, 16, 23] is caused by genetic mutations involving the same potassium channels that cause the LQTS but with an opposite effect [90–93]. In other words, in the SQTS there is excessive outflow of potassium currents, shortening the action potential duration and the effective ventricular refractory period. Distinguishing idiopathic VF from SQTS is not easy. Although the original cases of SQTS had extremely short QT intervals (QTc shorter than 300 ms [15, 16, 23] more recently described cases of genetically proven SQTS have QTc intervals of 360 ms [25]. Also, we recently showed that “relatively short” QT intervals (QTc <360 ms) are frequently observed in healthy males but are statistically more common in males with idiopathic VF [18]. Moreover, patients with idiopathic VF have normal QT intervals at normal heart rates, but their QT fails to lengthen as their heart rate slows down, leading to abnormally short QTc values during bradycardia [26, 27]. Finally, patients with SQTS and patients with idiopathic VF share the following clinically characteristics: (1) Both patient groups have similar spontaneous [5, 17] and inducible [3, 23] ventricular arrhythmias; (2) both patient groups appear to respond especially well to quinidine therapy [33, 52, 54, 94, 95]; (3) both patient groups are at risk for inappropriate ICD-shocks because of intracardiac T-wave oversensing [96, 97]. Patients with SQTS may be misdiagnosed as “idiopathic VF” if the QT interval is measured only during relatively rapid heart rates. This is because the main problem in the SQTS is failure of appropriate QT lengthening during bradycardia [23].
Brugada Syndrome
We estimated that one out five patients originally diagnosed as “idiopathic VF” have in fact Brugada syndrome [13] and very similar numbers were reported by others [98]. Moreover, if all patients with idiopathic VF undergo systematic testing with repeated electrocardiograms [99] and Holter recordings [100] with the right precordial electrodes placed at higher positions, as well as pharmacological challenge tests using sodium-channel blockers, as many as 40 % with idiopathic VF could be diagnosed as Brugada syndrome [101].
Short-Coupled Variant of Right Ventricular Outflow Tachycardia
The right ventricular outflow tract (RVOT) is the site of origin of the most common type of ventricular tachycardia (VT) occurring in patients without organic heart disease [33]. This RVOT-VT has a distinctive morphology (QRS complexes with left bundle branch block pattern and tall R waves in the inferior leads) and, in general, does not lead to hemodynamic decompensation. Therefore, RVOT-VT is considered a benign arrhythmia [33]. However, our group [65] and the group of Noda and Shimizu [66] recently described patients with otherwise typical “benign RVOT ectopy” who went on to develop spontaneous VF or polymorphic VT. It is not clear if patients with idiopathic VF and patients with this newly described form of “polymorphic VT from the RVOT” represent different aspects of one disease or two distinct disorders [102]. However, several characteristics differ among both groups: (1) otherwise typical monomorphic RVOT-VT is also seen in patients with malignant polymorphic RVOT-VT [65, 66] but is never seen in idiopathic VF. (2) Only 5 % of patients with “malignant polymorphic VT” have inducible VF [65, 66] by programmed ventricular stimulation, whereas the majority of patients with idiopathic VF have inducible VF [33, 52]. (3) the coupling interval of the ventricular extrasystoles initiating the malignant ventricular arrhythmias is invariably very short in idiopathic VF [3, 5] but is longer, varying from “relatively short” [65] to “normal,” [66] in the “polymorphic RVOT-VT” [103]. The last observation is consistent with the results of intracardiac mapping performed by Haissaguerre [35]: In that series, idiopathic VF originated from Purkinje fibers in 86 % of the patients and from the RVOT in the remaining 14 %. Again, the coupling interval of the extrasystoles initiating VF was longer for arrhythmias originating in the RVOT than for arrhythmias triggered by Purkinje fibers (355 ± 30 ms vs. 280 ± 26 ms, p < 0.01) [35].
Prognosis and Therapy of Idiopathic VF
The rate of recurrence of malignant ventricular arrhythmias in idiopathic VF, in the absence of therapy, is unacceptably high. At a mean follow-up of 6 years, more than 40 % of patients have recurrent VF and the risk is higher for those with normal electrocardiograms (that is, after excluding those with possible Brugada syndrome) [104]. In a recent series of patients with “truly idiopathic VF” (that is, excluding not only those with Brugada-type electrocardiogram at baseline but also those who developed ST-segment elevation when challenged with sodium channel blockers), the risk for recurrent VF was 39 % at 3.4 ± 2.3 years [51]. Therefore, once a diagnosis of idiopathic VF is made, some form of therapy is mandatory. Therapy may include ICD implantation, drug therapy with quinidine, radiofrequency ablation of the triggering focus or combinations of the above.
Drug Therapy with Quinidine
The very first patients with idiopathic VF described in the literature, back in 1929 [1] and 1949 [105], were treated with quinidine after multiple episodes of spontaneous polymorphic VT and VF were clearly documented. Both patients had an excellent response [1, 105]. In fact, a second publication reporting the long term follow-up of the patient initially reported in 1949, established that this patient eventually died of cancer at old age, without ever experiencing arrhythmia recurrence while on quinidine therapy for 40 years [106]. In 1987, Belhassen pioneered the therapy of idiopathic VF with EPS-guided quinidine after observing that VF is easily inducible at the baseline state but no longer inducible after quinidine therapy [2]. Of note, one of the patients included in that original report [52], has completed >25 uneventful years of electrophysiologic-guided therapy with amiodarone and quinidine after experiencing arrhythmic storms of VF in the absence of therapy and recurrent arrhythmic syncope on amiodarone alone [94].
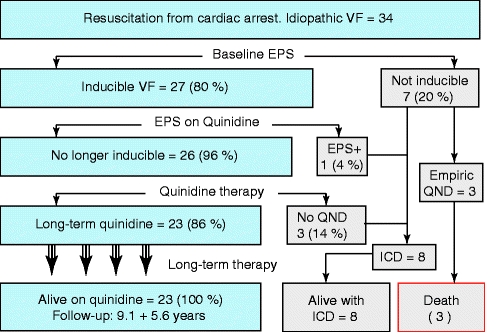
In 1990, when we first reviewed the topic of idiopathic VF [3], we found that the recurrence rate of cardiac arrest was high during therapy with other antiarrhythmic drugs (including amiodarone, beta-blockers or verapamil) [3]. The high-rate of arrhythmia recurrence with verapamil is worth noting because that drug was empirically proposed by Leenhardt and Coumel to treat the “short-coupled variant of torsade de pointes” [4], an entity that probably represents idiopathic VF. In contrast, we found that the recurrence rate with quinidine was nil [3]. By the time the ICD became commercially available, we continued to recommend quinidine as the sole therapy for appropriately selected patients with idiopathic VF, including patients resuscitated from spontaneous cardiac arrest [107]. Our criteria for quinidine therapy in VF survivors includes all the following: (1) diagnosis of idiopathic VF with or without Brugada syndrome; (2) inducible VF in the absence of drugs with programmed ventricular stimulation (Fig. 36.3); (3) no-inducible arrhythmias during oral quinidine therapy despite a very aggressive protocol of ventricular stimulation (Fig. 36.3) [2, 108, 109] (4) informed consent by a patient who is well informed of the risk and benefits of ICD and quinidine therapy for this disease [107]; (5) repeated assertion of drug compliance during long-term follow-up (compliance is assessed with quinidine serum levels and quinidine-effect on the QT interval). Our results using such approach were published in 1999 [53]. These results are shown in Fig. 36.5 and may be summarized as follows: Of 34 patients with idiopathic VF (all after resuscitation from cardiac arrest), 26 (80 %) had inducible VF at baseline electrophysiologic study and all but one of them were rendered non-inducible with quinidine. Side effects from quinidine led to discontinuation of quinidine therapy in 14 % of our patients. Nevertheless, 23 patients (2 out of 3 patients from the original cohort of cardiac arrest survivors) remained on quinidine therapy (without ICD back-up) and all are alive and completely free of arrhythmic symptoms that now exceeds 10 years. The long-term effectiveness of quinidine for preventing VF induction has been confirmed [110]. Relatively early in our experience, three patients who had negative electrophysiologic studies in the absence of drugs received empiric quinidine. All these patients died 4–8 years after the original VF episode. These 3 patients discontinued follow-up long before they died. Therefore, we do not know if the fatalities were due to poor compliance or due to drug failure and weather such failure was related to the fact that quinidine therapy for these particular patients was empiric and not guided by electrophysiologic studies (since these 3 patients were non-inducible at baseline). Nevertheless, we no longer recommend empiric use of quinidine for non-inducible patients after spontaneous cardiac arrest, a subgroup of patients for whom ICD implantation is mandatory. However, we have successfully used quinidine to control arrhythmic storms of ventricular fibrillations in patients who originally received an ICD either because of non-inducibility in the baseline EPS or because of (the extremely rare) persistence of inducibility while on quinidine therapy. The excellent response of VF storms in idiopathic VF with Brugada syndrome has also been repeatedly reported [60, 111–113]. Of note, the fatalities that occurred 4–8 years after the first VF event clearly demonstrate that patients with idiopathic VF remain at risk for fatal arrhythmia-recurrence even after long asymptomatic periods. Thus, long asymptomatic periods after the first VF episode should not be interpreted as resolution of an unidentified “myocarditis” and cannot be taken as a “good prognostic sign.”