Strong (risk increased by more than tenfold)
Fracture (hip or leg)
Hip or knee prosthesis
Major surgery (general)
Major trauma
Spinal cord injury
Moderate (risk increased by two to ninefold)
Arthroscopic knee surgery
Central venous lines
Chemotherapy
Chronic heart or respiratory failure
Hormone replacement therapy
Malignancy
Oral contraceptive therapy
Paralytic stroke
VTE in antecedents
Thrombophilia
Weak (odds ratio < 2)
Bed rest >3 days
Immobility due to sitting (e.g., prolonged travel)
Advanced age
Laparoscopic surgery
Obesity or overweight
Pregnancy/postpartum
Varicose veins
Risk Factors for VTE
Apart from traditional risk factors (Table 20.1), it worth mentioning less usual risk factors for VTE, which correspond to the classic atherosclerotic cascade. Many risk factors for VTE, such as dyslipidemia, obesity, hypertension, diabetes, and smoking, overlap with those for atherothrombosis. As a consequence, VTE is starting to be considered a “full-time member” of the cardiovascular syndrome “club” that includes coronary artery disease, peripheral artery disease, and cerebrovascular disease.
More evidence describing the continuum between VTE and arterial disease has appeared in past years [3]. Stein et al. [4] found a 2.5 increased risk for VTE in obese patients. In a prospective study of 250 women, the authors investigated which factors predispose to VTE. They found an increase in risk of 2.9-fold for obese subjects (body mass index >29 kg/m2), 1.9-fold for smokers, and 1.9-fold for those with hypertension [5]. Pradoni et al. [6] suggested a link between atherosclerosis and VTE; they discovered that patients with spontaneous veinous thrombosis had a higher incidence of carotid plaques than controls, translating into a 2.3-fold risk increase for VTE vs. controls. Subsequently, Spencer et al. [7] showed that idiopathic VTE is associated with increased risk of acute myocardial infarction among younger patient populations (ages 20–64 years). In addition, the risk of myocardial infarction and stroke was evaluated in a large population that included 25,199 patients with DVT, 16,925 patients with PE, and 163,566 controls [8]. During the subsequent 20 years of follow-up, presence of VTE was associated with a 20–40 % increase in risk for arterial cardiovascular events (for patients with DVT, the relative risks was 1.60 for myocardial infarction to 2.19 for stroke in the first year after the thrombotic event; for patients with PE, the relative risk was 2.60 for myocardial infarction and 2.93 for stroke). The fact that VTE and atherosclerosis are two analogous entities has recently been shown in a meta-analysis: patients with VTE have more asymptomatic atherosclerosis and more cardiovascular events than control subjects [9]. The risk of having a future VTE was 2.33 greater for obesity, 1.51 for hypertension, 1.42 for diabetes mellitus, 1.18 for smokers, and 1.16 for hypercholesterolemia. High-density lipoprotein (HDL) cholesterol was lower in subjects with events. Both low levels of HDL cholesterol and elevated fasting glucose have been found to double the risk of VTE in a cohort of 208 patients [10]. A large study of 20,374 middle-aged and elderly adults were followed for more than 12 years for incident VTE, and results showed that metabolic syndrome was associated with an 1.84-fold increased risk for VTE, a result largely attributable to abdominal obesity [11].
Diet also influences the occurrence of thrombotic events. A total of 14,962 middle-aged adults participated in a prospective trial to evaluate the role of dietary intake in the development of DVT or PE [12]. Surprisingly, a diet including more plant food and fish and less red and processed meat was associated with a lower incidence of VTE.
Hormonal replacement therapy and contraception have been linked to atherothrombotic events and venous thrombotic disease. Contraceptives, especially those that contain third-generation progestins, increase the risk of VTE [13]. The Women’s Health Initiative randomized trial enrolled subjects receiving estrogen-plus-progestin hormone replacement therapy and showed a twofold increase in the risk of VTE compared with those in the placebo group [14].
An interesting question, that of whether venous thromboembolism is related to psychosocial factors, was answered in a study dating from 2008 [15]. The authors found that persistent stress and low occupational class were independently related to future PE but not to DVT.
Pathophysiology
The three keywords that describe both venous thrombosis and atherothrombosis are inflammation, systemic/local hypercoagulability, and endothelial dysfunction. Inflammation plays an important role in the pathogenesis of this entity; elevated C-reactive protein (a sensitive marker of systemic inflammation) has been linked to an increased risk of VTE. A total of 10,505 patients enrolled in the ARIC (Atherosclerosis Risk in Communities) study showed that elevated C-reactive protein is independently associated with increased risk of VTE [16]. Increased values of systemic inflammatory markers (C-reactive protein, fibrinogen, and factor VIII) are especially found in patients who had an unprovoked DVT or PE compared with those with secondary VTE [17]. Genetic anomalies of interleukin (IL)-1β and IL-10 genes also influence the risk of idiopathic VTE [18]. The JUPITER (Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) trial showed that 20 mg/day of rosuvastatin reduced the rate of symptomatic VTE by 43 % in patients with elevated C-reactive protein and low-density lipoprotein (LDL) cholesterol levels <130 mg/dl compared with placebo [19].
Hypercoagulability anomalies (which together with endothelial lesions and venous stasis form Virchow’s triad) include inherited thrombophilias (activated protein C resistance due to factor V Leiden mutation; prothrombin gene mutation; congenital dysfibrinogenemia; deficiencies of protein C, protein S, and antithrombin III), hyperhomocysteinemia (most often acquired because of dietary folate deficiency but also can be inherited because of a deficiency in methylenetetrahydrofolate reductase; has been associated with both VTE and atherothrombosis), and antiphospholipid antibody syndrome (APLAS; an acquired thrombophilia that increases the risk for both VTE and arterial thromboembolism).
Diagnosis
The initial assessment for determination of DVT is the clinical probability assessment. For suspected DVT, the Wells score has been validated and has well-established criteria (Table 20.2). A score ≥2 indicates that the probability of DVT is likely, and a score of <2 indicates that the probability of DVT is unlikely. For PE, a Wells score >4 indicates PE is likely, and a score of ≤4 indicates PE is unlikely (Table 20.3). We can also use the simplified or revised Geneva score (Table 20.4).
Table 20.2
Wells score for DVT diagnosis
Clinical characteristics | Score |
|---|---|
Active cancer | +1 |
Paralysis or plaster immobilization | +1 |
Bed rest >3 days or major surgery in the last 4 weeks | +1 |
Localized tenderness along the distribution of the deep venous system | +1 |
Entire leg swollen | +1 |
Calf swelling >3 cm when compared with asymptomatic leg | +1 |
Pitting edema | +1 |
Collateral superficial veins (nonvaricose) | +1 |
Previously documented deep vein thrombosis | +1 |
Alternative diagnosis at least as likely as deep vein thrombosis | −2 |
Clinical probability | |
Unlikely | <2 |
Likely | ≥2 |
Table 20.3
Well’s score for PE diagnosis
Clinical characteristics | Score |
|---|---|
Hemoptysis | +1 |
Cancer | +1 |
Previous pulmonary embolism or deep venous thrombosis | +1.5 |
Heart rate >100/min | +1.5 |
Recent surgery or immobilization | +1.5 |
Clinical signs of deep venous thrombosis | +3 |
Alternative diagnosis less likely than that of pulmonary embolism | +3 |
Clinical probability | |
Low | <2 |
Intermediate | 2–6 |
High | >6 |
Table 20.4
Revised and simplified revised Geneva scores for PE diagnosis
Clinical characteristics | Revised score | Simplified score |
|---|---|---|
Age >65 years | +1 | +1 |
Active malignant condition | +2 | +1 |
Surgery or fracture within 1 month | +2 | +1 |
Hemoptysis | +2 | +1 |
Previous deep vein thrombosis or pulmonary embolism | +3 | +1 |
Unilateral lower-limb pain | +3 | +1 |
Heart rate 75–94/min | +3 | +1 |
Pain on lower deep venous palpation and unilateral edema | +4 | +1 |
Heart rate >94/min | +5 | +1 |
Clinical probability | ||
Low | 0–3 | 0–1 |
Intermediate | 4–10 | 2–4 |
High | >10 | ≥5 |
Symptoms such as dyspnea, cough, or chest pain are present in the majority of patients with PE. Additional symptoms include hemoptysis and syncope. Specific signs for PE are tachypnea (>20 respiratory cycles/min), tachycardia (>100 beats/min), signs of DVT, fever (>38.5 °C), and cyanosis.
Chest x-rays are nonspecific but can exclude other causes of dyspnea and chest pain. Electrocardiographic findings could show signs of RV overload such as inversion of T waves in V1-V4, QR pattern in V1, complete or incomplete right bundle branch block, or the classic S1Q3T3.
Negative highly sensitive D-dimer assays can exclude PE in patients with low or moderate clinical probability, whereas moderately sensitive arrays can exclude PE only in patients with a low clinical probability.
Compression ultrasonography (CUS) can be used either as a backup procedure to reduce a false-negative result when using single-detector computer tomography (SDCT) or can be used in patients that have contraindications to contrast dye or radiations (Fig. 20.1).
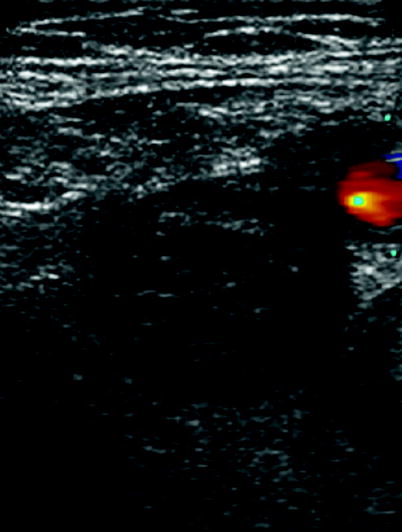

Fig. 20.1
Compression ultrasonography showing a thrombosed femural vein (middle plane) and patent femural artery (right side)
Ventilation-perfusion scintigraphy is very safe for excluding a PE if it is normal. A high probability of PE establishes the diagnosis with a high degree of probability; additional tests may be considered in selected patients with a low clinical probability.
Single-detector computer tomography (SDCT) and multi-detector CT (MDCT) are diagnostic if a thrombus is evident at least at a segmental level, whereas incertitude exists regarding treatment of a sub-segmental defect (Figs. 20.2 and 20.3). Patients with non-high clinical probability can have only MDCT or combined SDCT plus CUS for diagnostic purposes. Further testing is needed in patients with negative MDCT and high clinical probability.
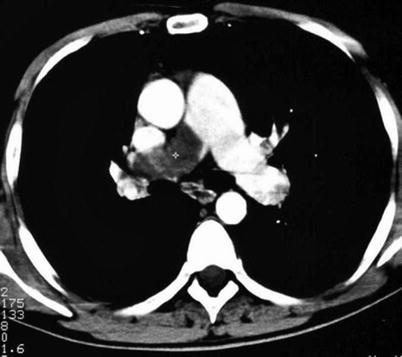
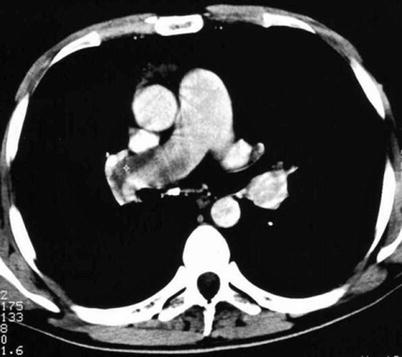

Fig. 20.2
Computer tomography plane showing a thrombosed proximal right pulmonary artery

Fig. 20.3
Computer tomography plane showing a thrombosed proximal right pulmonary artery
Among invasive diagnostic exams, even though pulmonary angiography is the gold standard in the diagnosis of PE, it is rarely used because of its invasive nature. Diagnostic criteria for PE include direct evidence of a thrombus, visible filling defect, and other indirect signs (slow flow contrast, regional hypoperfusion, and delayed or diminished pulmonary venous flow). Venography is also less used today (Figs. 20.4 and 20.5).
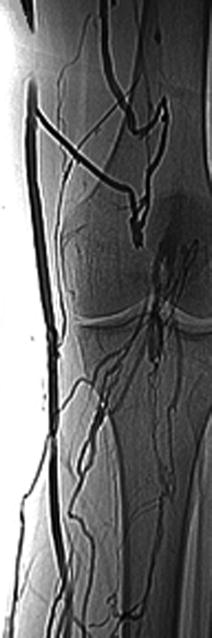
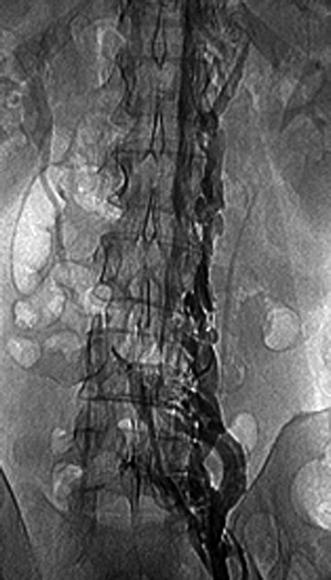

Fig. 20.4
Venography of the left thigh showing thrombosed great saphenous vein

Fig. 20.5
Venography of an extended thrombosed iliac vein
Echocardiography is especially useful in emergency management decisions. The absence of RV overload/dysfunction excludes PE as a cause of hemodynamic instability in a patient with hypotension or shock (Figs. 20.6, 20.7, 20.8, 20.9, 20.10, and 20.11). It has a very important role in stratifying patients with non-high risk PE into intermediate and low-risk categories. Echocardiographic criteria of RV dysfunction include RV hypokinesis and dilatation, RV size >30 mm at annulus, tricuspid insufficiency of >2.8 m/s, a ratio of RV/left ventricle >1, paradoxical septal systolic motion, and a pulmonary acceleration time <90 ms.
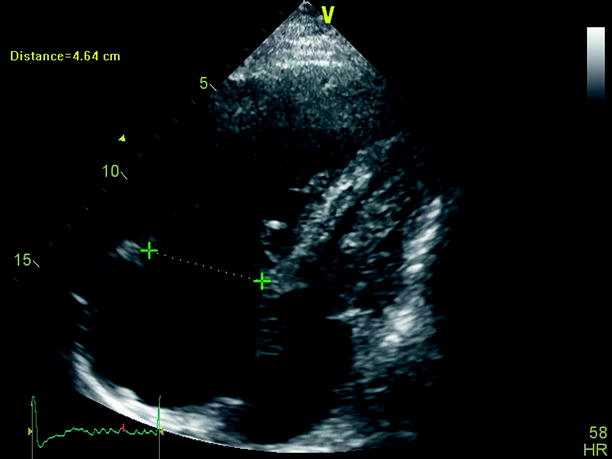
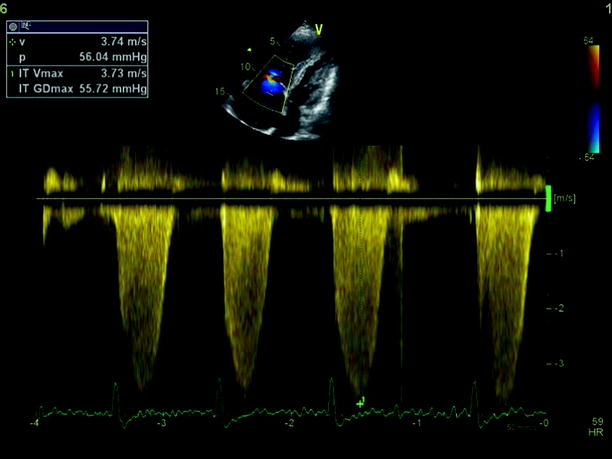
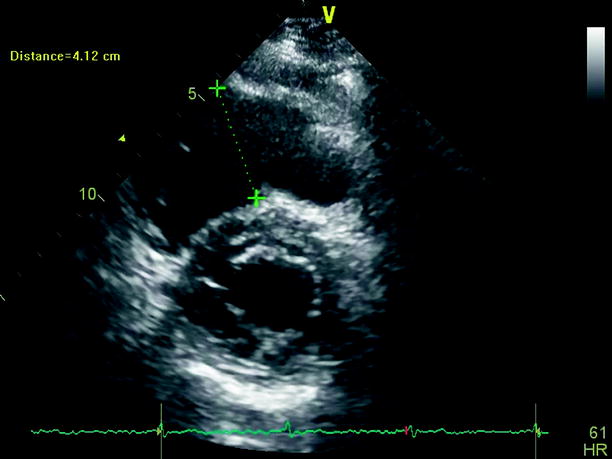
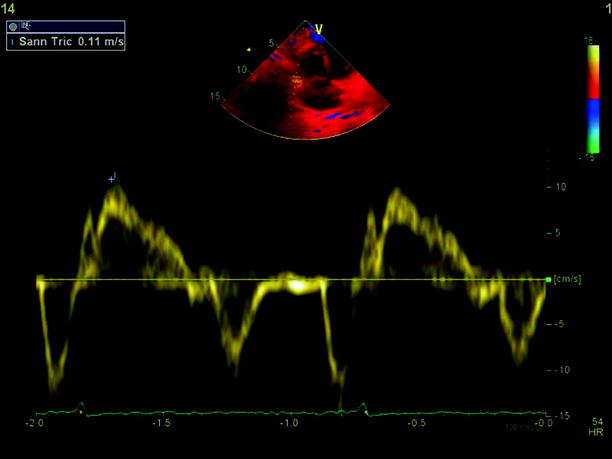
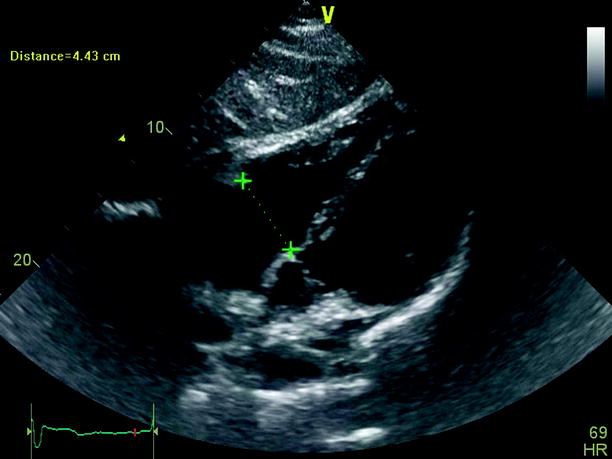
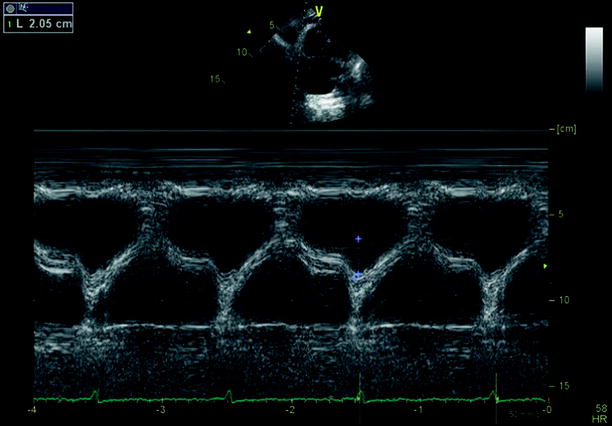

Fig. 20.6
Subcostal view of a dilated right ventricle in the context of chronic thromboembolic pulmonary hypertension

Fig. 20.7
CW Doppler on the tricuspid valve showing elevated right ventricle-right atrium gradient

Fig. 20.8
Parasternal short-axis view at mitral valve level showing a dilated right ventricle in the context of a hemodinamic unstable pulmonary embolism

Fig. 20.9
Preserved systolic function using pulsed tissue doppler of the right ventricle free wall in the context of pulmonary embolism

Fig. 20.10
Subcostal view showing a dilated right ventricle in the setting of a hemodinamic unstable pulmonary embolism

Fig. 20.11
Preserved systolic function using the tricuspid annular plane systolic motion (TAPSE) of the right ventricle free wall in the context of chronic thromboembolic disease
Complications
(a)
Complications of DVT include the post-thrombotic syndrome (Figs. 20.12 and 20.13) (evolves in 20–50 % of patients and could result in lifelong limb pain, swelling, heaviness, edema, and leg ulcers) [27] and PE.
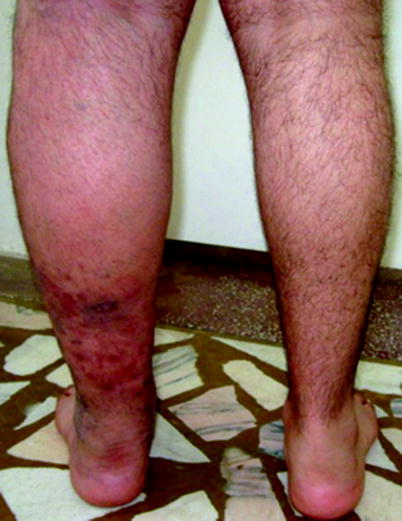
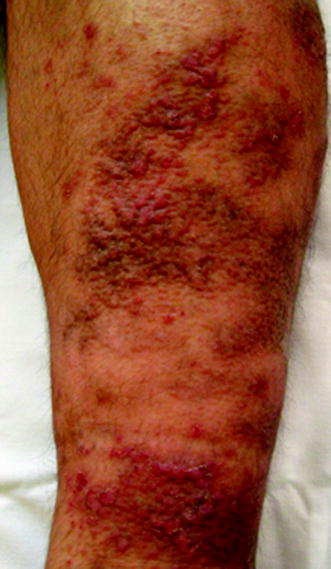

Fig. 20.12
Post-thrombotic syndrome associated with oedema and cutaneous trophic disorder (left leg)

Fig. 20.13
Post-thrombotic syndrome associated with cutaneous trophic disorder
(b)
Complications of PE include RV dysfunction, hemodynamic instability, and chronic thromboembolic hypertension. In the acute phase, stratification of PE includes high-risk PE and non-high-risk PE.
High-risk PE is a life-threatening condition in which short-term mortality exceeds 15 %. It includes PE in association with shock or hypotension (systolic blood pressure <90 mmHg or a pressure drop >40 mmHg for >15 min if not caused by new-onset arrhythmia, hypovolemia, or sepsis).
Non-high-risk PE is further categorized into intermediate (3–15 % short-term risk of mortality) and low-risk PE (<1 % short-term mortality). Intermediate-risk PE is defined by the presence of RV dysfunction defined by the presence of at least one of the following: echocardiographic findings (previously mentioned), RV dilatation at CT, BNP or NT-proBNP elevation, increased right heart pressure at right heart catheterization, and positive cardiac troponin T or I.
Treatment
Once DVT has been diagnosed, treatment goals are: symptom relief, prevention of embolization and recurrence, control of thrombus progression during the acute phase in order to clear away the risk of an immediate, possibly fatal pulmonary embolism, control of acute and chronic pulmonary and peripheral venous hypertension, and control of relapsing disease in the intermediate and long-term course. Treatment of VTE is composed of three periods: the acute phase (days), the intermediate phase (weeks or months), and the long-term period (months or years).
Acute Treatment
In patients with DVT or stable PE, either unfractionated heparin or low-molecular-weight heparin (LMWH) can be used safely (except in case of severe renal impairment for LWMH use). Monitoring anti-Xa activity is useful in patients with severe renal failure, in pregnancy, and in obese patients (to be done 4 h after the morning injection). Monitoring of unfractionated heparin is done 4–6 h after the bolus injection and 3 h after each adjustment, aiming for an activated partial thromboplastin time of 70–90 s. Table 20.5 shows standard doses of LMWH. There is no benefit of immobilization for the clinical outcome of patients with PE. Wearing stockings markedly reduces the incidence of post-thrombotic syndrome.
Table 20.5
Curative doses of LMWH and fondaparinux for PE treatment
Dose | Interval | |
|---|---|---|
Enoxaparin | 1.0 mg/kg | Every 12 h |
or 1.5 mg/kga | Once dailya | |
Tinzaparin | 175 U/kg | Once daily |
Fondaparinux | 5 mg (body weight <50) | Once daily |
7.5 mg (body weight 50–100 kg) | ||
10 mg (body weight >100 kg) |
In patients with hemodynamic unstable PE, intravenous thrombolysis is mandatory, followed by unfractionated heparin (bolus of 80 U/kg and subsequent infusion of 15–18 U/kg/h). The most used thrombolytic protocols are shown in Table 20.6 [28–30]. Contraindications to thrombolysis are shown in Table 20.7. Thrombolysis could be used in patients with intermediate-risk PE after thorough consideration of conditions that increase bleeding risk. Surgical pulmonary embolectomy can be performed in patients who may need cardiopulmonary resuscitation, in patients with contraindications to thrombolysis, and in those with a patent foramen ovale and intracardiac thrombi. Hemodynamic support including vasopressors, and mechanical ventilation may be needed in severe cases.
Table 20.6
Thrombolytic doses for pulmonary embolism
Streptokinase [28] | 250,000 IU as a loading dose over 30 min, followed by 100,000 IU/h over 12–24 h. Accelerated regimen: 1.5 million IU over 2 h |
Urokinase [29] | 4,400 IU/kg as a loading dose over 10 min, followed by IU/kg/h over 12–24 h |
Accelerated regimen: 3 million IU over 2,100 mg over 2 h or 0.6 mg/kg over 15 min (maximum dose 50 mg) |
Table 20.7
Contraindications to fibrinolytic therapy
Absolute contraindications a |
Hemorrhagic stroke |
Stoke of unknown origin at any time |
Ischemic stroke in preceding 6 months |
Central nervous system damage or neoplasms |
Recent major trauma/surgery/head injury (within preceding 3 weeks) |
Gastrointestinal bleeding within the last month |
Known bleeding |
Relative contraindications |
Transient ischemic attack in preceding 6 months |
Oral anticoagulant therapy |
Pregnancy or within 1 week postpartum |
Non-compressible punctures |
Traumatic resuscitation |
Refractory hypertension (systolic blood pressure >180 mmHg) |
Severe liver disease |
Infective endocarditis |
Active peptic ulcer |
Intermediate Phase Treatment and Chronic Treatment
After the acute phase, oral anticoagulation is mandatory. It can be done either by vitamin K antagonists (VKAs) or by newer anticoagulants. VKAs such as warfarin have several limitations, such as a slow onset of action, frequent monitoring of INR because of the limited therapeutic index, food and drug interactions, inter-individual dosing differences, and warfarin resistance. The only apparent advantages of VKAs over newer anticoagulants are the possibility to administer an antidote in case of overdose and its use independent of renal failure.
Newer anticoagulants are available and are ready to use in VTE (Table 20.8). The main advantages over VKAs include: fixed-dose administration, no INR testing, and no interactions with food or drugs.
Table 20.8
Comparative properties of thrombin and factor Xa inhibitors in comparison to warfarin
Warfarin | Rivaroxaban | Apixaban | Dabigatran | Edoxaban | |
|---|---|---|---|---|---|
Target | Vitamin K | Factor Xa | Factor Xa | Factor IIa | Factor Xa |
Prodrug | No | No | No | Yes | No |
Bioavailability (%) | >95 | >57–86 | >49 | 6.5 | 50 |
T (max) (h) | 72–96 | 2–4 | 1–4 | 1.25–3 | 1–2 |
Half-life (h) | 40 | 9–13 | 8–15 | 12–14 | 9–11 |
Monitoring | INR-adjusted | Not needed | Not needed | Not needed | Not needed |
Administration in VTE | Once daily | 15 mg bid for 3 weeks followed by 20 mg od | 10 mg bid for 1 week followed by 5 mg bid | 150 mg bid | 60 mg od |
Metabolism and elimination | CYP 2C9, 3A4, 1A2 | CYP3A4; 33 % renal, 66 % fecal | CYP3A4; 75 % fecal, 25 % renal | 80 % renal, 20 % fecal | 35 % renal |
Drug interactions | CYP 2C9, 1A2, and 3A4 | CYP 3A4 inhibitor | CYP 3A inhibitor | P-GP inhibitor | P-GP inhibitor |
P-GP inhibitor | P-GP inhibitor |
Rivaroxaban is an oral direct inhibitor of factor Xa [33] that inhibits factor Xa in a concentration-dependent manner via a rapid and reversible binding. It reduces the rate of development of VTE in patients after total hip or knee arthroplasty vs. LMWH with no significant differences in risk of bleeding [34–37]. Compared with enoxaparin, it reduces the costs associated with drug administration for prophylaxis and treatment of VTE events. Also it reduces the incidence of symptomatic VTE [38].
Apixaban is a reversible active direct inhibitor of factor Xa that can also be administered orally (fixed oral dose may replace LMWH combined with vitamin K antagonists in the treatment of DVT) [39]. Compared with LMWH, apixaban was more effective for prevention of VTE as compared with LMWH without enhancing bleeding risk after knee and hip replacement [40, 41].
Dabigatran etexilate is a competitive reversible oral anticoagulant that inhibits thrombin directly. It has the potential to replace traditional anticoagulants for prevention of VTE in patients who have undergone elective total hip or knee replacement surgery [42, 43].
Oral anticoagulants should be started as soon as possible, preferably on the first day if a stable PE has been diagnosed, and should aim for a target INR (international normalized ratio) of 2–3 when VKAs are used.
Oral anticoagulants should be continued for 3 months if a reversible factor has been identified (surgery, trauma, medical illness, estrogen therapy, pregnancy, etc.; see Table 20.1).
Patients with thrombophilia (deficit of protein C or S, lupus anticoagulant, homozygous for factor V Leyden, or homozygous for PTG 20210A) are candidates for chronic oral anticoagulant treatment as recurrence is high. At this time, there is no clear benefit of chronic VKA treatment in patients heterozygous for factor V Leyden or heterozygous for PTG 20210A.
Patients with provoked or apparently unprovoked VTE should benefit from chronic oral anticoagulants treatment, especially if any of the factors in Table 20.9 are present. Also, if the bleeding risk is low, lifelong anticoagulation is advised.
Table 20.9
Factors that increase recurrence risk in idiopathic VTE
Immobilization |
Cancer |
Chronic obstructive pulmonary disease |
Family history |
Male gendera |
Overweight, obesitya |
Low levels of apolipoprotein AI and high-density lipoprotein cholesterola |
Proximal DVTa |
Symptomatic PEa |
Elevated D-dimer levels after discontinuing anticoagulationa |
Failure to recanalize leg veins after anticoagulation for DVTa |
Treatment for chronic thromboembolic hypertension is pulmonary endarterectomy and should be advised when severe symptoms and RV failure occur.
For cancer patients, LMWH and especially dalteparin are indicated for the first 3–6 months of a proximal DVT and/or PE at an initial dose of 200 U/kg subcutaneously once daily; afterwards oral anticoagulants should be continued indefinitely or until cancer is cured.
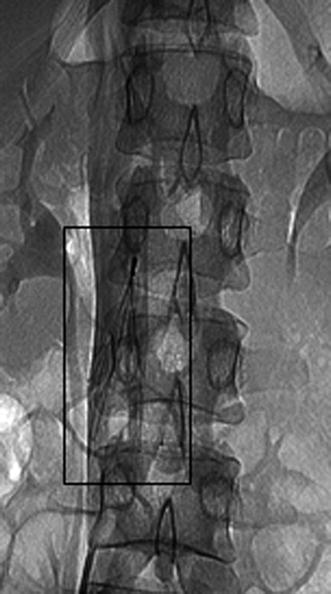
The rationale for caval filter insertion (Fig. 20.14) is to lower the chance of pulmonary embolism originating from a proven proximal DVT (usually with dimensions more than 4 mm). According to the latest American Heart Association guidelines on VTE (2011), venous filters are indicated when there is an absolute contraindication to anticoagulation and when there is a high risk of VTE recurrence while on anticoagulation and evidence of active bleeding complications requiring termination of anticoagulation therapy. Relative contraindications include large, free-floating iliofemoral thrombus in high-risk patients, propagating iliofemoral thrombus while on anticoagulation, patients with significant fall risk, and chronic PE in patients with pulmonary hypertension. As soon as oral anticoagulants can be introduced, they should be removed because of increased complication rates (insertion site thrombosis in 10 % of cases, recurrent DVT in 20 % of cases, post-thrombotic syndrome in 40 %, and occlusion of the inferior vena cava in 22 % of patients at 5 years). Whatever the reason for filter insertion, temporary filters should be favored over permanent filters.
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


