15 Hypoplastic Left Heart Syndrome
I. CASE
A. Fetal echocardiography findings
1. The fetal echo reveals situs solitus of the atria, levocardia, left aortic arch, and increased cardiothoracic ratio (0.45).
2. The LV is severely hypoplastic, with atretic mitral and aortic valves.
3. There is evidence of increased echogenicity at the ventricular walls and papillary muscles of the mitral valve. The LV shows endocardial fibroelastosis with decreased function. The RV is dilated, with large tricuspid and pulmonary valves that were not dysplastic and had no significant regurgitation.
4. The transverse and distal aortic arch are of normal size and by color and pulsed Doppler there was flow reversal into the distal arch. Usually, the distal arch is the largest aspect of the arch in a patient with hypoplastic left heart syndrome (HLHS), presumably because it receives the greatest amount of flow.
5. The RV Tei index (myocardial performance index) is normal (0.4).
6. There are no signs of hydrops fetalis.
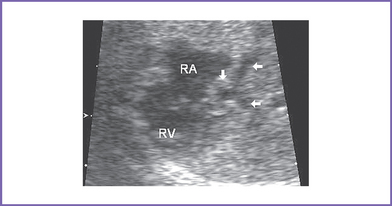
7. The interatrial septum is intact (no flow across the atrial septum) (Fig. 15-1). The left atrium (LA) is mildly enlarged, with bowing of the interatrial septum toward the right atrium (RA).
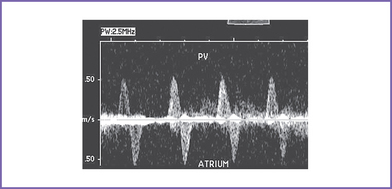
8. The pulmonary veins in the LA are decompressed through a vertical vein, which is obstructed at the site of the left pulmonary artery and left bronchus (mean gradient, 6-7 mm Hg). The pulmonary venous flow is abnormal, with a significant a wave reversal in systole and no forward flow in early ventricular diastole, suggesting severely elevated LA pressure.
9. The pulsatility index is increased in the proximal pulmonary artery, suggesting elevated fetal pulmonary vascular resistance (PVR).
D. Fetal management and counseling
1. Amniocentesis was performed before referral and showed normal fetal karyotype and negative fluorescent in situ hybridization (FISH) analysis for 22q11 microdeletion.
c. Follow-up included serial antenatal studies at weekly intervals initially and then every 2 to 4 weeks.
F. Neonatal management
a. Compassionate care with either no treatment from delivery or withdrawal of treatment once the diagnosis has been confirmed.
c. Staged palliative surgery (Norwood procedure).
d. Cardiac transplantation, particularly in patients with cardiac pathology that can complicate single ventricle palliation, such as significant RV dysfunction or severe tricuspid valve regurgitation.
a. PGE1 infusion to keep the ductus open to provide a pathway to the systemic circulation and improve the body perfusion.
b. Administration of oxygen is normally not advisable in neonates with HLHS because this can cause a decrease in the PVR with increased pulmonary blood flow that further steals from the systemic circulation. However, in a neonate with severe restriction of the atrial septum and LA hypertension, oxygen administration can contribute to an improvement in the systemic oxygen levels.
c. Blood gas revealing severe metabolic acidosis and slightly decreased Po2, a characteristic finding of HLHS, could be due to poor cardiac output. However, babies with severe LA hypertension due to atrial level restriction are both severely cyanotic (Pao2 < 25-30 mm Hg) and metabolically acidotic. In this setting, therapeutic balloon atrial septostomy can help decompress the LA.
d. The hematocrit should be maintained above 45% to increase the oxygen-carrying capacity.
e. Usually, volume and inotropic support (such as milrinone 0.5 μg/kg/min infusion) may be indicated to improve ventricular function.
a. Single ventricle palliation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree