7 Hypertrophic Cardiomyopathy
Background
• Hypertrophic cardiomyopathy (HCM) is defined as the presence of left ventricular hypertrophy occurring in the absence of a cardiac or systemic disorder (e.g., valvular aortic stenosis or systemic hypertension).1
• Initially regarded as a rare disorder, the prevalence of HCM in the general population is now estimated to be approximately 1 in 500.
• HCM is a heterogeneous disorder with a spectrum of clinical findings, ranging from completely asymptomatic individuals to severely affected patients.
• The symptoms of HCM are largely attributable to diastolic filling abnormalities, dynamic left ventricular outflow tract (LVOT) obstruction, atrial or ventricular arrhythmias, and/or myocardial ischemia.
Overview of Echocardiographic Approach
• Echocardiography plays a vital role in the evaluation and management of patients with HCM (Box 7-1).
Step-by-Step Approach to the Evaluation of HCM
Step 1: Establish the Diagnosis of HCM
Step 2: Exclude Other Causes of Increased Wall Thickness
• Left ventricular hypertrophy caused by a genetic defect of the cardiac sarcomeric proteins needs to be distinguished from other important causes (Box 7-2).
Box 7-2 Differential Diagnosis of HCM on Echocardiography
Key Points
• The main categories in the differential diagnosis of left ventricular increased wall thickness are the following:
• Common causes of left ventricular hypertrophy are systemic hypertension and other conditions causing pressure-overload hypertrophy, such as valvular aortic stenosis, fibrous subaortic stenosis, and coarctation of the aorta.
• These non-monogenetic forms of left ventricular hypertrophy typically have a concentric (rather than asymmetrical) pattern of hypertrophy.
• However, they may be associated with a degree of focal septal hypertrophy (especially in the elderly) and/or the presence of systolic anterior motion (SAM) (see Step 4 below).
Athlete’s Heart3
• Wall thickness may be ≥ 13 mm (up to 15 to 16 mm) in 2% of elite athletes, raising the possibility of underlying mild HCM.
Storage Diseases4
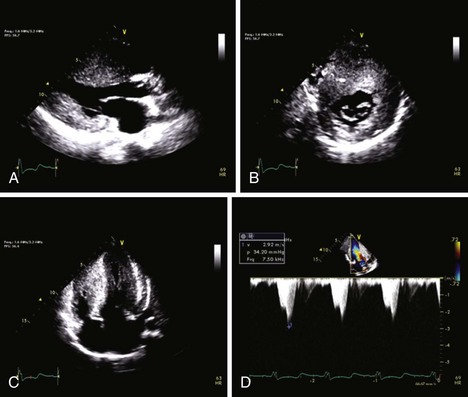
• The intramyocardial accumulation or infiltration of abnormal metabolic products in various storage diseases may mimic HCM on echocardiography (Figure 7-1).
• Fabry’s disease is an X-linked recessive disorder of glycosphingolipid metabolism due to a deficiency of the lysosomal enzyme α-galactosidase A.
• Patients with Fabry’s disease may have multisystem involvement, especially of the skin, kidneys, nervous system, and heart.
• Genetic testing for the causative gene in Fabry’s, the α-galactosidase (GLA) gene, is also available.
• Mutations of genes regulating glycogen metabolism may also result in increased wall thickness on echocardiography.
• Mutations of the regulatory γ2 subunit of AMP-activated protein kinase (PRKAG2) gene cause a glycogen storage cardiomyopathy, which may be associated with ventricular preexcitation.
• Mutations of the lysosome-associated membrane protein 2 (LAMP2) gene (X-linked) cause Danon’s disease.
• Danon’s disease is a lysosomal glycogen storage disease characterized clinically by cardiomyopathy, electrophysiologic abnormalities, myopathy, and variable mental impairment.
Key Points
• The prevalence of Fabry’s disease has ranged from 3% in all male patients with left ventricular hypertrophy to as much as 6% in male patients with presumed late-onset HCM (defined as diagnosis at age ≥ 40 years).
• In a cohort of 24 patients with increased wall thickness and ventricular preexcitation, mutations of the PRKAG2 and LAMP2 genes were detected in 29% and 17% of patients, respectively.
• Clinical features distinguishing patients with LAMP2 mutations from patients with HCM caused by sarcomeric gene mutations included the following: male sex, early onset of disease (<17 years of age), ventricular preexcitation on electrocardiography, severe concentric hypertrophy, and elevation of two serum enzymes: creatine kinase (CK) and alanine aminotransferase (ALT).
Infiltrative Cardiomyopathies
• Conditions such as cardiac amyloidosis or sarcoidosis are associated with increased wall thickness and may occasionally mimic HCM on echocardiography.
• In general, the infiltrative cardiomyopathies usually have the following distinguishing features compared with HCM:
Septal Hypertrophy in the Elderly
• It may be difficult to distinguish between elderly patients with HCM and those with hypertensive heart disease.
• Terms that have been used to describe this finding are “sigmoid septum,” “septal bulge,” and “discrete upper septal hypertrophy.”
• It is controversial whether this finding should be considered a subtype of HCM or whether it represents a benign anatomic variant.
Step 3: Assess Pattern and Severity of Left Ventricular Hypertrophy
• 2D echocardiography can accurately detect and characterize the degree and extent of hypertrophy in patients with HCM.
Key Points
• Asymmetrical septal hypertrophy (septal/posterior [inferior] wall thickness ratio > 1.3) is the classic echocardiographic pattern detected in patients with HCM.
Anatomic Imaging
Acquisition
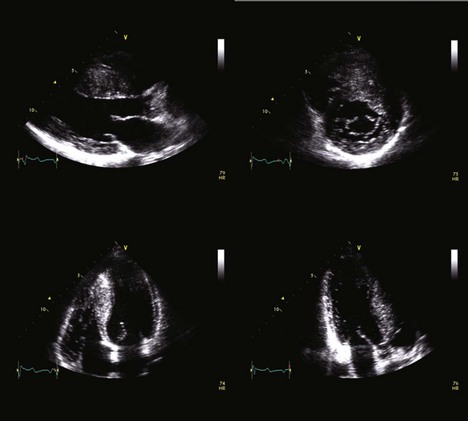
• Multiple transthoracic echocardiographic (TTE) views are necessary to assess the extent and severity of left ventricular hypertrophy (Figure 7-2).
• Measurements of the left ventricular wall segments (anteroseptal, inferoseptal, anterior, anterolateral, inferolateral, inferior) should be made in the parasternal short-axis views at the basal and midventricular levels.
• The apical septal, anterior, lateral, and inferior segments should be measured in the parasternal short-axis views at the apical level.
• Hypertrophied segments usually have either similar or slightly increased echogenicity in comparison with myocardial segments with normal wall thickness.
• In addition, diffuse bright echoes may be seen throughout the myocardium and may give a ground-glass appearance.
Analysis
• Septal involvement may be limited to the basal level or the hypertrophy may extend to the left ventricular apex.
Key Points
• Two different septal morphologic subtypes have been described:
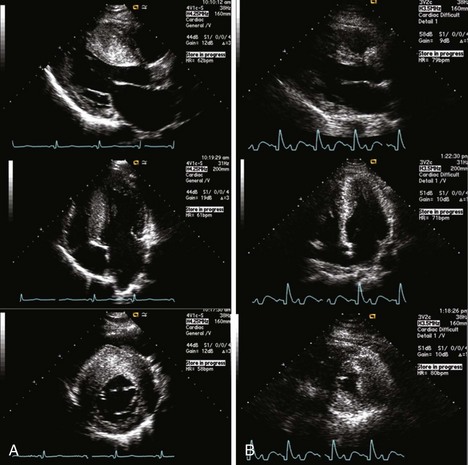
• “Reverse septal curvature”—the maximal thickness of the septum occurs at the midventricular level, predominant midseptal convexity toward the left ventricular cavity, with the cavity itself having an overall crescent shape (Figure 7-3A)
• Young patients with HCM characteristically demonstrate the reverse septal curvature subtype, whereas older patients typically have the basal septal morphologic subtype.
• Patients with HCM and asymmetrical septal hypertrophy may have hypertrophy confined to the septum, or they commonly have hypertrophy of both the ventricular septum and the free wall (anterolateral wall segments).
• The distribution of hypertrophy can be in any pattern and can involve any myocardial segment (including the right ventricle).
• Other uncommon morphologic variants of HCM include asymmetrical apical HCM (predominant hypertrophy at the apical segments) and a concentric pattern of left ventricular hypertrophy.
• An ultrasound contrast agent should be administered when nonenhanced images are suboptimal for definitive diagnosis, such as in patients with suspected apical HCM.6
Severity of Left Ventricular Hypertrophy
• Since there is considerable variability in the thickness of the different septal and posterior wall segments in patients with HCM, the conventional calculations of left ventricular mass are not applicable in these patients.5
• Therefore, a number of echocardiographic indices have been developed to measure the distribution, extent, and/or burden of left ventricular hypertrophy.7
• Wigle’s score: Incorporates the degree of septal thickness at the basal anteroseptum, the extent of septal involvement, and the presence or absence of anterolateral wall involvement (Table 7-1).
• Wall thickness (Spirito-Maron) index: Obtained by adding the maximal wall thickness (at either the basal or midventricular level) of four left ventricular regions (anterior septum, posterior septum, lateral wall, and posterior wall).
• Maximal left ventricular wall thickness: The most clinically relevant measurement is the determination of the maximal thickness of all the left ventricular myocardial segments. The presence of massive left ventricular hypertrophy, defined as a wall thickness of ≥ 30 mm, has considerable implications for patients’ management and prognosis (see “Key Measurements for Predicting Prognosis in Patients with HCM” below).1
TABLE 7-1 EXTENT OF HYPERTROPHY ACCORDING TO ECHOCARDIOGRAPHIC POINT SCORE
| Extent of Hypertrophy | Points |
|---|---|
| Septal thickness, mm (basal third of septum) | |
| 15–19 | 1 |
| 20–24 | 2 |
| 25–29 | 3 |
| >30 | 4 |
| Extension to papillary muscles (basal two thirds of septum) | 2 |
| Extension to apex (total septal involvement) | 2 |
| Anterolateral wall extension | 2 |
| Maximum total | 10 |
Other Morphologic Subtypes of HCM
Apical HCM
• Prominent findings of patients with apical HCM include the presence of giant negative T waves (≥10 mm) in the precordial leads on electrocardiography and an “ace of spades” configuration to the left ventricle seen on imaging studies.
Step 4: Determine if There Is LVOT Obstruction
• Studies of cohorts of patients with HCM have shown that approximately 25% of patients have LVOT obstruction at rest.
• One recent study has suggested that 70% of patients with HCM have either resting or provocable LVOT obstruction.
• Early M-mode echocardiographic studies demonstrated the abnormalities in morphology of the LVOT and in the mitral apparatus that contribute to the development of dynamic LVOT obstruction.
• Rapid early left ventricular ejection out of the LVOT also contributes to the development of SAM and LVOT obstruction.
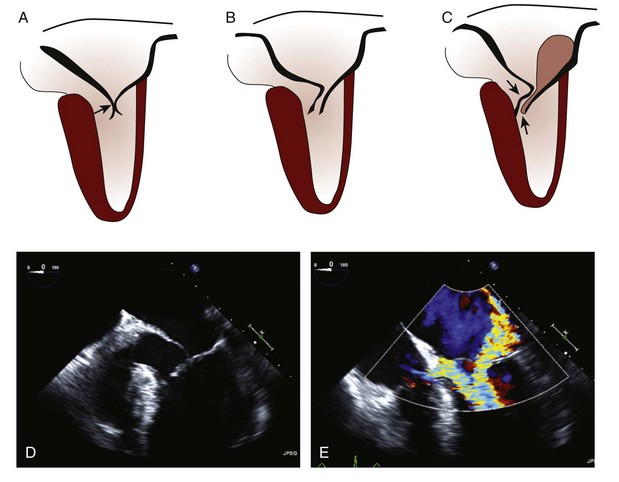
• Morphologic features of HCM that contribute to SAM and LVOT obstruction include narrowing of the LVOT by the following:
Key Points
• Although the nature of the hydrodynamic forces on the anterior mitral leaflet remains controversial, it is believed that the distal anterior mitral leaflet is subjected to Venturi and/or drag forces.2
• Therefore, SAM occurs and the tip of the anterior mitral leaflet typically develops a sharp anterior and superior angulation, leading to mitral leaflet–septal contact in early to midsystole (Figure 7-4).
• Simultaneous cardiac catheterization and M-mode echocardiographic studies have shown that mitral leaflet–septal contact occurs almost simultaneously with the onset of the LVOT pressure gradient.
• Furthermore, there is a linear relationship between the time of onset of SAM and the severity of LVOT obstruction.
• SAM of the anterior leaflet leads to an interleaflet gap, which results in a posteriorly directed jet of mitral regurgitation (see Step 5 below).