Fig. 38.1
Depicted is MRI 4ch view with a missing LV, restrictive atrial septum, and anterior positioned single right ventricle
38.6 Technique (Step-by-Step)
Hybrid procedure consists of bilateral pulmonary arterial banding (bPAB) and duct stenting performed as part of the approach during an open chest – beating heart scenario or as an elective transcatheter approach in a spontaneous breathing, sedated newborn with or without an additional atrioseptostomy [2, 3].
Currently, PAB is performed surgically by an open-chest approach without cardiopulmonary bypass. In our center, the percutaneous transcatheter approach is usually performed by femoral vein or arterial access (Chap. 25).
38.6.1 Step-by-Step Approach
Step 1 depends on the clinical condition of the newborn; surgical bPAB is performed as a high-urgency approach in a newborn with HLHS and pulmonary runoff, but even as the first step of the hybrid approach in a well-conditioned newborn with a still balanced pulmonary to systemic circulation (Fig. 38.2a–d). Balanced circulation in HLHS is present by a Qp-Qs of 1, as in a patient with an arterial (SaO2) venous (SvO2) oxygen saturation difference of about 20 % meaning SaO2 of 80 % and SvO22 of 60 %, respectively. It has to be mentioned that bPAB should only be performed as the first step in newborns with free or only slightly obstructed interatrial communication with a minimal pressure gradient and in newborns with wide-open ductus arteriosus achieved by low dosages (2–10 ng/kg/min) of prostaglandin.
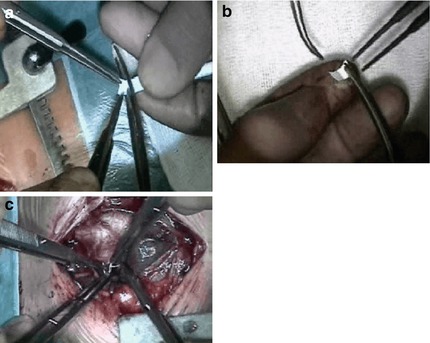

Fig. 38.2
Open-chest approach for bilateral pulmonary banding; (a) shows cutting of a 3.5 mm PTFE tube. (b) The opened “tube” strip. (c) Suturing the strip around the right pulmonary artery
In newborns with a body weight of more than 3 kg, a 3.5 mm PTFE shunt is cut receiving a strip of only 2 mm, which is used for bilateral PAB; in lower body weight the same shunt is sutured that the lumen is less than 3.5 mm, and in a premature or newborn with less than 2.5 kg, a 3 mm GORE-TEX shunt is cut and used for bilateral pulmonary banding. The surgical procedure does not consume more than 20 min; the time in the operation theater takes 2–3 h.
In Step 2, following the surgical bPAB, the patient is transferred to the cardiac intensive care unit, and in elective cases immediate extubation is the most important therapeutic goal. Cardiac-supporting drugs are mostly not necessary, if the SaO2 is less than 85 %. In still high SaO2 values, re-banding has to be considered, but mostly not necessary because after extubation in spontaneous breathing patient, the SaO2 is mostly further decreasing. However, lowering systemic vascular resistance by milrinone 0.2–1 μg/kg × min might be of additional benefit as well as long-acting oral vasodilative drugs. In our institution the ACE inhibitor lisinopril is used together with the highly specific ß-blocker bisoprolol both in low dosages of 0.05 mg/kg once per day. The dosage of the ß-blocker is adapted to the heart rate.
Considering the “Giessen hybrid” approach, elective duct stenting is performed following surgical bilateral PAB in an immediate extubated patient.
In Step 3, if the decision is made for the percutaneous completion of hybrid stage I, manipulation of the interatrial septum (IAS) is performed if necessary. Atrioseptostomy should regularly be performed before duct stenting. The techniques for atrioseptostomy range between a Rashkind procedure and static balloon dilatation with or without cutting-balloon technique or an immediate IAS stenting. In the past, we used premounted balloon expandable stents (Genesis or Valeo stents) with a high risk of stent slipping during the pullback procedure of bad deflated balloons. The change to self-expandable open-cell stents (OptiMed) of 8 × 12 or 8 × 15 mm sinus-SuperFlex-DS made this approach safe and very easy. In addition, the need for only a 4 F sheath to deliver these stents allows even using an unusual access as a transhepatic approach with low risk and good success (Fig. 38.3).
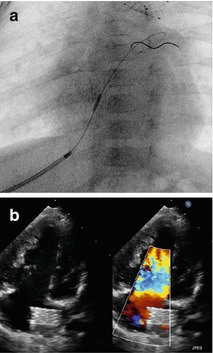

Fig. 38.3
(a, b) Stent placement within the intact atrial septum (IAS) by transhepatic approach (a); the thin struts of the sinus-SuperFlex-DS (8 × 15 mm) deliverable through a 4 F sheath are almost not visible; the stent positioned in the IAS is better shown by echocardiography
In Step 4, for duct stenting a multipurpose catheter is advanced through a 4 F sheath placed in the femoral artery. The catheter is guided by BMW-0.014″ floppy coronary wire from the descending aorta through the duct in the pulmonary artery. After hemodynamic determinations by pullback pressure measurements from the pulmonary and from the aortic arch to the descending aorta (DAO), biplane (RAO 30° and 90° lateral) angiography is performed to analyze the ductus morphology including the smallest width and length and to delineate the junction of the descending aortic arch and the ductus. Based on these data, stent width and length are chosen. As mentioned in Chap. 25, the diameter of the utilized stent is chosen at least 1–2 mm above the minimal measured duct diameter, but in any case exceeding the diameter of the descending aorta. In most patients with HLHS, a stent with a width of 8 mm is recommended.
In Step 5, the venous approach for duct stenting is described in Chap. 25. Here, the approach through the femoral artery is shortly summarized. For stent placement, the soft coronary wire (BMW, Abbott) is exchanged for a stiff 0.014 coronary wire (S’port, Abbott). Utilizing even the novel developed self-expandable stents with a CE mark for duct stenting in newborns with HLHS (OptiMed, Karlsruhe, Germany), duct stenting is currently performed mostly by the arterial access (Fig. 38.4a–d).
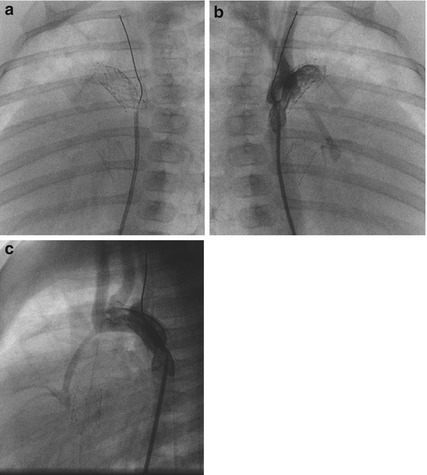

Fig. 38.4
(a–c) Depicted are self-expandable sinus-SuperFlex-DS of 8 × 18 mm positioned in the ductus arteriosus and one 8 × 15 mm within the IAS as well as a 5 × 9 mm sinus-Repo-DS at the side of an aortic coarctation in a newborn with HLHS (MA, AA). Lateral projections are shown in (a, c) and RAO 30° view in (b)
In Step 6, after preparing the pre-packed self-expandable sinus-SuperFlex-Duct Stent (SSF-DS) and flushing of the 4 F delivery system, the system in total is carefully advanced over the stiff S’sport from the DAO to the PA.
In Step 7, based on the biplane angiography, a marker for stent placement is necessary. The stent is expanded under fluoroscopy by controlled pullback of the covering part of the delivery system until the stent is fully expanded; in some patients, short cine scenes are necessary to visualize sufficiently the very thin struts of the open-cell designed stent.
In Step 8, the lateral 90° plane is used to control the stent position at the end of the pulmonary artery. Right anterior oblique (RAO 30°) is the plane of choice to observe the exact stent position in relation to the junction of the duct to the descending aorta.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


