Fig. 5.1
The ITACA (International Tailored Chemotherapy Adjuvant Trial), a phase III multicenter randomized trial comparing adjuvant pharmacogenomic-driven chemotherapy versus standard adjuvant chemotherapy in completely resected stages II–IIIA NSCLC
From Advance to Early-Stage NSCLC: The Epidermal Growth Factor Receptor Molecular Pathway
To date, the use of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) in patients with EGFR mutations is currently the standard of care in stage IV NSCLC. The role of these agents has become of clinical interest also in the perioperative setting in early stage, although the evidence is not conclusive yet. A potential benefit in early stage was suggested in a retrospective study from the Memorial Sloan Kettering Cancer Center (MSKCC). In the study 167 patients with stages I–III EGFR mutant NSCLC were retrospectively considered, and 56 patients who received neo-adjuvant or adjuvant EGFR-TKI were compared to the other 111 patients who did not receive TKI. Patients treated with EGFR-TKI had a 2-year disease-free survival (DFS) rate of 89 %, as compared with 72 % for patients not treated with TKI (p = 0.06), even if the 2-year OS in both groups (≥90 %) was not statistically different [42]. In another retrospective study from MSKCC, 22 patients who recurred after adjuvant EGFR-TKI treatment were identified, of whom 11 were retreated with EGFR-TKI and 8 responded for a median duration of 10 months [67]. Data from these retrospective studies hypothesize that adjuvant TKI therapy may not increase cure rate but simply delay recurrences.
Currently, data on the use of gefitinib as an adjuvant treatment are not conclusive [33, 47] as they are based on retrospective assessments and subgroup analyses of prospective studies. Data from a small Chinese trial of 60 patients with resected stage IIIA-N2 NSCLC and positive for EGFR-sensitizing mutations, postoperatively treated with either 4 cycles of adjuvant carboplatin and pemetrexed (PC) or the same chemotherapy followed by 6 months of gefitinib (PC-G), showed improvement in the gefitinib versus the control arm for median DFS (39.8 vs. 27.0 months, p = 0.014, HR = 0.37), but no significant difference in OS was observed (median 41.6 months vs. 32.6 months, p = 0.066). The 2-year PFS and OS rates were 78.9 and 92.4 % in PC-G group and 54.2 and 77.4 % in PC alone group, respectively [101]. The potential efficacy of erlotinib in the adjuvant setting is currently investigated by the RADIANT trial (Fig. 5.2), already completely accrued [64]. This is a phase III trial which targeted 945 patients with stages I–IIIA NSCLC whose tumors have EGFR protein expression by immunohistochemistry (IHC) or increased EGFR gene copy number by fluorescence in situ hybridization (FISH). Following surgical resection and optional adjuvant chemotherapy, patients were randomized 2:1 to erlotinib for 2 years or placebo. The initial results are expected soon. The SELECT II phase trial [64] is another adjuvant study currently ongoing. Thirty-six patients with surgically resected stages IA-IIIA NSCLC harboring activating EGFR mutations were enrolled and treated with erlotinib 150 mg/daily for 2 years after completion of any standard adjuvant chemotherapy and/or radiotherapy. Ten patients required one dose reduction for toxicities. After a median follow-up of 2.5 years, the 2-year DFS from enrollment was 94 % (95 % CI 80 %, 99 %), 10 patients recurred, and most were still responsive to subsequent EGFR-TKI therapy [65]. This trial was subsequently expanded to 100 patients and has been completely enrolled, with results expected within 1–2 years (Fig. 5.3). Other studies, involving the administration of new- and old-generation TKI, are underway in the neo- and/or adjuvant setting in early-stage NSCLC with EGFR mutations. A definitive trial evaluating erlotinib and crizotinib, a molecular-targeted agent for EML4-ALK translocated NSCLC, in molecularly selected patients is set to start in the United States in the near future (ALCHEMIST trial). While the trial will initially focus on patients with EGFR mutations and EML-ALK fusion, respectively, it is designed to allow for inclusion of additional targets as active agents for them will be identified.
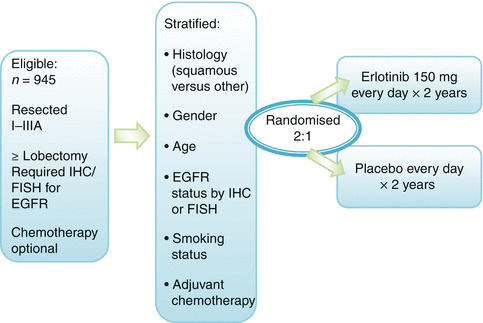
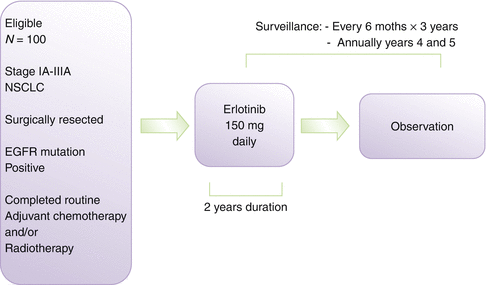

Fig. 5.2
The RADIANT trial RADIANT Randomized Double-Blind Trial in Adjuvant NSCLC with Tarceva, EGFR epidermal growth factor receptor, FISH fluorescence in situ hybridization, IHC immunohistochemistry

Fig. 5.3
The SELECT trial
Specific Genes Involved in the Activity of Specific Cytotoxic Agents
Proteins of the nucleotide excision repair pathway are thought to repair DNA damage caused by platinum agents. The excision repair cross-complementing (ERCC) gene family reduces damage to DNA by nucleotide excision and repair [98]. ERCC1 activity may be assessed through different methods including immunohistochemistry (IHC) or automated AQUA technology or, alternatively, quantitative real-time polymerase chain reaction (qRT-PCR). There are no data about the superiority of one approach versus the other because a comparison among these techniques is missing [17, 22]. Initially the predictive role of ERCC1 was assessed in patients with advanced NSCLC-treated cisplatin-based doublets [13, 58]. Resected NSCLC (stages IA to IIIB) patients with high messenger RNA (mRNA) ERCC1 expression (>50 unitless ratio) had a better survival outcome when compared to patients with low ERCC1 (median OS, 94.6 vs. 35.5 months; p = 0.01), and mRNA ERCC1 expression was an independent and significant predictor of favorable outcome [92]. The predictive role of ERRC1 was investigated by immunohistochemistry in a subgroup of 761 patients enrolled in a large adjuvant chemotherapy trial. The absence or low expression of ERCC1 was associated with a benefit from cisplatin-based adjuvant chemotherapy (test for interaction, p = 0.009) and with a significantly prolonged DFS and OS among patients with ERCC1-negative tumors (HR for death, 0.65; 95 % CI, 0.50–0.86; p = 0.002) unlike those with ERCC1-positive tumors. The prognostic value of ERCC1 was confirmed in the control group with a significantly higher 5-year OS among patients with ERCC1-positive tumors (HR, 0.66; 95 % CI, 0.49–0.90; p 0.009) [1, 2]. Also the evaluation by AQUA confirmed that low ERCC1 scores were marginally prognostic (HR = 0.77 for high vs. low scores, p = 0.10) [6].
The predictive value of ERCC1 was enhanced by the concurrent evaluation of MutS homolog 2 (MSH2), a major active component of the mismatch repair system. Patients with double-negative tumors experienced a greater benefit from chemotherapy [44].
However, in a recent further analysis on a larger number of samples, these findings could not be reproduced, likely due to the fact that currently available monoclonal antibodies could not distinguish among the four ERCC1 protein isoforms, whereas only one isoform produced a protein that had full capacities for nucleotide excision repair and cisplatin resistance [28]. Thus, ERCC1 is not currently being tested in routine practice to select adjuvant chemotherapy.
ERCC1 expression has been more rarely investigated in the context of neo-adjuvant studies. ERCC1 is a prognostic factor after platinum-based neo-adjuvant chemotherapy following surgical resection (p < 0.05) [45]. In another neo-adjuvant study, mRNA ERCC1 levels were correlated with an objective response (OR) from platinum-based chemotherapy. A significant correlation was observed between the ERCC1 expression level and the chance to achieve an objective response during platinum-based chemotherapy (p < 0.05), but not with the formation of local or distant metastases [61]. In 113 elderly patients with NSCLC, ERCC1 expression was correlated with the outcomes of neo-adjuvant chemotherapy. The median survival time was 53 months in ERCC1-negative patients as compared to 37 months in those patients with ERCC1-positive tumors. ERCC1 expression level in the tumor tissue and TNM stages were independent factors that affected the prognosis of these patients (p < 0.05). Data from this study indicate that neo-adjuvant chemotherapy may induce ERCC1 expression in the tumor and the objective response rate of neo-adjuvant chemotherapy may be reduced in NSCLC patients with high ERCC1 expression [53].
Breast cancer susceptibility gene 1 (BRCA1) is involved in transcription couples nucleotide excision repair (NER) and functions as a differential regulator of chemotherapy-induced apoptosis induced by antimicrotubule drugs, such as taxanes and vinca alkaloids, while conferring resistance to DNA-damaging agents, including platinum agents [19]. In chemotherapy-naive patients with early-stage NSCLC, overexpression of BRCA1 mRNA was associated with poor survival, and BRCA1 mRNA expression was an independent factor predicting survival at the multivariate analysis [76].
A recent meta-analysis has sought to clarify the predictive role of BRCA1. In platinum-based studies, low/negative BRCA1 expression was associated with better objective response rate (ORR) (HR = 1.70), longer OS, and event-free survival (EFS) (HR = 1.58, and HR-2.39 for OS and EFS, respectively). Patients treated with paclitaxel and that expressing a high/positive BRCA1 have better ORR (HR = 0.41), while OS and EFS were not evaluated because of the insufficient data available [106]. However, data are conflicting. In a Spanish phase II feasibility study of adjuvant chemotherapy in completely resected stages II–IIIA NSCLC, treatment was customized based on BRCA1 mRNA levels and high BRCA1 received docetaxel, while low BRCA1 were treated with a cisplatin doublet; OS did not differ between the treatment arms [16].
RAP80 is another DNA repair protein in which low expression was also suggested to influence PFS and OS when associated with low levels of BRCA1. In addition a close correlation with BRCA1 and RAP-80 expression was identified as an independent predictor for OS [75]. The role of RAP-80 should be assessed also in the early stage as potential gene to address, together with BRCA1, treatment customization. An ongoing Spanish cooperative study is addressing the question in the context of a large phase III study comparing standard adjuvant treatment versus customization according to the expression of these two genes.
Ribonucleotide reductase M1 (RRM1) is the regulatory component of an essential enzyme that catalyzes the reduction of ribonucleoside diphosphates to the corresponding deoxyribonucleotides [77]. RRM1 is a major predictor of disease response to gemcitabine, being its predominant target, as well as platinum [25]. Several trials in early stage have investigated the prognostic [111] and predictive value of RRM1 in patients treated with gemcitabine plus cisplatin [5, 7]. RRM1 expression was significantly and inversely correlated with disease response, though not with survival [74]. Recent data from a retrospective study have indicated that ERCC1 expression and RRM1 expression were not prognostic of tumor recurrence and OS in patients with completely resected NSCLC, either with or without adjuvant chemotherapy [95].
Some studies reported that ERCC1 expression is closely related to RRM1 and BRCA1 levels [77, 78, 109], with concordant levels in 70–80 % of cases [29].
Thymidylate synthase (TS) catalyzes the conversion of deoxyuridine monophosphate (dUMP) to (deoxy) thymidine monophosphate (TMP), which requires oxidization of tetrahydrofolate to dihydrofolate. High tumoral levels of TS have been associated with resistance to 5FU [43, 52, 90]. In advanced NSCLC lower median mRNA and protein TS levels predict response and survival in non-squamous NSCLC treated with pemetrexed, while higher median levels in squamous histology and small cell lung cancer are associated with an inferior efficacy in these two histotypes [12, 13, 62, 81].
In chemotherapy-naive patients with resected early-stage NSCL, TS is a prognostic factor. In one study high TS mRNA, but not protein, expression was significantly associated with adverse disease-free survival (DFS), and in the other study high TS expression, as determined by AQUA but not by qRT-PCR, predicted improved OS [89, 110].
Few prospective randomized clinical trials are currently testing the pharmacogenomics hypothesis using these genes to personalize adjuvant chemotherapy. As a representative example, the ITACA (International Tailored Chemotherapy Adjuvant) trial (Fig. 5.3) is a randomized phase III trial comparing adjuvant pharmacogenomic-driven chemotherapy based on ERCC1 and TS assessment by qRT-PCR versus standard adjuvant chemotherapy in completely resected stages II–IIIA NSCLC. This study is almost completed for the accrual, and more than 700 patients are already randomized [66].
Miscellaneous Biomarkers
In few studies K-ras mutation was associated with a poor prognosis in early NSCLC patients [50] but not in others, although some data suggest a possible negative predictive role [11, 96]. In an Italian adjuvant study, a subgroup of 227 patients with non-squamous tumors were investigated for the presence of K-ras mutation, and at the univariate, but not at the multivariate analysis, K-ras mutation was associated with shorter survival [83].
The role of TP53 gene mutation is quite intriguing, but the sensitivity and the predictive role of the evaluation of p53 by IHC are not optimal [35]. In fact, in the adjuvant setting the independent unfavorable prognostic role of p53 (IHC) but not of the TP53 [84] was confirmed.
In advanced NSCLC ßTubIII expression may predict response and outcome in patients treated with tubulin-binding agents [24, 85]. High ßTUBIII expression was shown to be an independent adverse predictor of recurrence-free survival [86]. The prognostic value was confirmed retrospectively in patients enrolled in another adjuvant study [72] and more recently in a neo-adjuvant study [108].
The upregulation of cyclin-dependent kinase inhibitor 1B, p27 Kip1 , leads to de novo resistance to platinum agents with a benefit survival in patients with p27Kip1-negative tumors [27, 73]. Also cyclin D2 has been associated with poor recurrence-free survival in patients in stage III NSCLC treated with surgery with or without adjuvant chemotherapy [49]. It has been observed that in early-stage NSCLC, insulin-like growth factor receptor (IGF1R)/EGFR FISH + and IGF1R/EGFR IHC + were associated with shorter disease-free survival (p = 0.05 and p = 0.05, respectively)[59]. In chemotherapy-naive patients with resected stages I–III NSCLC, a high hepatocyte growth factor receptor (c-MET) was an independent adverse prognostic factor mainly in squamous histotype [31]. Similarly, in patients with early-stage NSCLC, HER2 expression was associated with poorer prognosis especially in stages IB and IIA diseases [104]. New biomarkers are gaining ground as higher expression of CXCR7 that is associated with metastatic progression and poor DFS in patients with stage I NSCLC [41] or as CXCR4 that, if negative, seems to have a possible prognostic significance [93]. Recently, a set of genes with altered methylation status was identified in stage I NSCLCs, some of which associated with survival [18, 57]. Methylated BRCA1 can be a potential biomarker that predicts the prognosis after curative resection of stage I NSCLC [36]. Studies in early-stage NSCLC have reported an association between vascular endothelial growth factor (VEGF) overexpression and progression or poor survival [4, 54].
Conclusion
To date, the customization of perioperative treatment of early-stage NSCLC remains an investigational issue for clinical trials, and it is not yet ready for prime time. The literature data are often conflicting as related to retrospective assessments or the use of different assessment techniques not directly comparable. Results of ongoing randomized clinical trials are largely awaited to gain enough evidence for potential use in the routine setting, and, therefore, currently the systemic treatment individualization is still confined to clinical characteristics of the patient, histological findings, and stage of the tumor.
References
1.
2.
Arriagada R, Auperin A, Burdett S, et al.; NSCLC Meta-analyses Collaborative Group. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small cell lung cancer: two meta-analyses of individual patient data. Lancet. 2010;375(9722):1267–77.
3.
Arriagada R, Bergman B, Dunant A, et al.; International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350(4):351–60.
4.
Baillie R, Carlile J, Pendleton N, et al. Prognostic value of vascularity and vascular endothelial growth factor expression in non small cell lung cancer. J Clin Pathol. 2001;54:116–20.PubMedCrossRefPubMedCentral
5.
6.
Bepler G, Olaussen KA, Vataire AL, et al. ERCC1 and RRM1 in the international adjuvant lung trial by automated quantitative in situ analysis. Am J Pathol. 2011;178:69–78.PubMedCrossRefPubMedCentral
7.
Bepler G, Sommers KE, Cantor A, et al. Clinical efficacy and predictive molecular markers of neoadjuvant gemcitabine and pemetrexed in resectable non-small cell lung cancer. J Thorac Oncol. 2008;3:1112–8.PubMedCrossRefPubMedCentral
8.
Boeri M, Verri C, Conte D, et al. MicroRNA signatures in tissues and plasma predict development and prognosis of computed tomography detected lung cancer. Proc Natl Acad Sci U S A. 2011;108:3713–8.PubMedCrossRefPubMedCentral
9.
10.
Butts CA, Socinski M, Mitchell P, et al. START: A phase III study of L-BLP25 cancer immunotherapy for unresectable stage III non-small cell lung cancer. J Clin Oncol. 2013;31(No 15s): abstr 7500, 458s.
11.
Capelletti M, Wang XF, Gu L, et al. Impact of KRAS mutations on adjuvant carboplatin/paclitaxel in surgically resected stage IB NSCLC: CALGB 9633. J Clin Oncol. 2010;28(No 15s): abstr 7008, 516s.
12.
13.
14.
Chang HY, Sneddon JB, Alizadeh AA, et al. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2004;2(2):E7.PubMedCrossRefPubMedCentral
15.



