To assess the proportion and long-term outcomes of patients with idiopathic dilated cardiomyopathy and potential indications for implantable cardioverter-defibrillator before and after optimization of medical treatment, 503 consecutive patients with idiopathic dilated cardiomyopathy were evaluated from 1988 to 2006. A total of 245 patients (49%) satisfied the “Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) criteria,” defined as a left ventricular ejection fraction of ≤0.35 and New York Heart Association (NYHA) class II-III on registration. Among these, 162 (group A) were re-evaluated 5.4 ± 2 months later with concurrent β-blockers and angiotensin-converting enzyme inhibitor use. Of the 162 patients, 50 (31%) still had “SCD-HeFT criteria” (group A1), 109 (67%) had an improved left ventricular ejection fraction and/or New York Heart Association class (group A2), and 3 (2%) were in NYHA class IV. Of the 227 patients without baseline “SCD-HeFT criteria” (left ventricular ejection fraction >0.35 or NYHA class I), 125 were evaluated after 5.5 ± 2 months. Of these 227 patients, 13 (10%) developed “SCD-HeFT criteria” (group B1), 111 (89%) remained without “SCD-HeFT criteria” (group B2), and 1 (1%) had worsened to NYHA class IV. The 10-year mortality/heart transplantation and sudden death/sustained ventricular arrhythmia rate was 57% and 37% in group A1, 23% and 20% in group A2 (p <0.001 for mortality/heart transplantation and p = 0.014 for sudden death/sustained ventricular arrhythmia vs group A1), 45% and 41% in group B1 (p = NS vs group A1), 16% and 14% in group B2 (p = NS vs group A2), respectively. In conclusion, two thirds of patients with idiopathic dilated cardiomyopathy and “SCD-HeFT criteria” at presentation did not maintain implantable cardioverter-defibrillator indications 3 to 9 months later with optimal medical therapy. Their long-term outcome was excellent, similar to that observed for patients who had never met the “SCD-HeFT criteria.”
Since the publication of the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) and the DEFibrillators In Non-Ischemic cardiomyopathy Treatment Evaluation Trial (DEFINITE), the treatment with implantable cardioverter-defibrillator (ICD) for the primary prevention of sudden death (SD) has been extended to patients with nonischemic idiopathic dilated cardiomyopathy (IDC), who have a left ventricular (LV) ejection fraction (EF) of ≤0.35 and who are classified as New York Heart Association (NYHA) functional class II or III (“SCD-HeFT criteria,” class I indication, level of evidence B). The appropriate timing for ICD implantation, however, is still uncertain. Current guidelines suggest that an ICD should be considered in addition to medical therapy, but many patients are treated without evidence-based indications, mainly because of newly diagnosed heart failure and before treatment optimization. In our study, we evaluated the proportion of patients with and without potential indications for ICD implantation at presentation and the long-term prognosis of patients with initial ICD indications but who improved after optimization of medical treatment. Finally, we compared the long-term outcome of “improved” patients to those maintaining “SCD-HeFT criteria” and those who never met “SCD-HeFT criteria.”
Methods
In our study, we evaluated all patients with IDC who were not taking β blockers and had data recorded in the Heart Muscle Disease Registry of Trieste, Italy, from January 1, 1988 to April 30, 2006. After our first assessment, optimization of medical treatment was achieved with β blockers, if tolerated, and angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers at the highest tolerated dosage. Although the data were retrospectively analyzed, the patients were prospectively included in the Registry, and follow-up visits were regularly scheduled, according to the policy of our institution, after about 6 months during the first year and every 12 months afterwards. For the purpose of the present analysis, patients with NYHA class IV, those who died before a second evaluation and patients with a second evaluation performed >9 months after the first visit were excluded from the study population.
The patients were usually referred to our center by general practitioners or by primary or secondary care hospitals. Those already taking ACE inhibitors were included, because these drugs were usually started earlier, by general practitioners, since the late 1980s.
A physical examination, 12-lead electrocardiogram, transthoracic echocardiogram, exercise stress test, 24-hour Holter monitoring, and invasive hemodynamic study were performed. All patients >35 years old underwent coronary angiography to exclude significant coronary artery stenosis (>50% in a major vessel). Until 1996, the patients routinely underwent endomyocardial biopsy to exclude active myocarditis. Thereafter, biopsies were performed only in those patients presenting with recent (<6 months) onset of heart failure and/or clinical history suggestive of active myocarditis. The diagnosis of IDC was made when the LVEF was decreased (<0.50) in the absence of any other known cause of cardiac disease. In the present analysis, only patients evaluated after 1988 were included. This date was chosen because since that year, according to the policy of our institution, β blockers (metoprolol or, later, carvedilol) have been tested and started in all tolerating patients.
Medical treatment of IDC was considered optimal when the maximum tolerated dose of β blockers and ACE inhibitors was administered. To achieve this condition, low doses of β blockers were tested and slowly uptitrated to the highest dosage tolerated within 2 to 3 months. ACE inhibitors were introduced and/or the dosages increased before the second evaluation. The daily dosage of ACE inhibitors and β blockers reached at the second evaluation were reported as enalapril and carvedilol equivalents, respectively.
Antialdosterone agents were given only for potassium-sparing intent and, after the publication of the Randomized Aldactone Evaluation Study (RALES), to patients with persisting severe heart failure despite the optimization of medical treatment.
Amiodarone was administered in the presence of frequent and/or symptomatic ventricular or supraventricular arrhythmias. No other antiarrhythmic drugs were given.
According to the guidelines available before 2006 and our internal policy, the patients were not treated with an ICD for primary prevention during the first year after diagnosis. The patients with previous major ventricular arrhythmias (MVA) were excluded from the analysis.
The patient population was divided into 2 prespecified groups. Group A included those who satisfied the “SCD-HeFT criteria” and group B, those with a LVEF >0.35 and/or NYHA class I on presentation. According to the response to medical treatment, patients belonging to groups A and B were further divided into 4 subgroups:
- Group A1
patients with “SCD-HeFT criteria” both on presentation and at the second evaluation
- Group A2
patients with “SCD-HeFT criteria” on presentation but with a LVEF >0.35 and/or NYHA class I at the second evaluation
- Group B1
patients with a LVEF >0.35 and/or NYHA class I on presentation but with “SCD-HeFT criteria” after 6 months
- Group B2
patients with a LVEF >0.35 and/or NYHA class I both on presentation and at the second evaluation
The study design, data analysis, and reporting were performed according to the “Strengthening the Reporting of Observational Studies in Epidemiology” (STROBE) guidelines. The study was performed in accordance with the guidelines set by the Declaration of Helsinki and with the local legal requirements.
All values are reported, as appropriate, as the mean ± SD or number and percentage. Repeated measures of continuous variables were compared by paired t test. For binary variables, the McNemar test was used. Survival curves were calculated according to the Kaplan-Meier method, and the comparison between curves was performed with the log-rank test. All results were considered as statistically significant when p <0.05. The entire analysis was performed using the Statistical Package for Social Sciences package, version 13.0 (SPSS, Chicago, Illinois).
Results
From January 1, 1988 to April 30, 2006, 503 consecutive patients were recorded in the Heart Muscle Disease Registry of Trieste, Italy (see flow diagram in Figure 1 ) . About ½ of patients satisfied the “SCD-HeFT criteria” at the first evaluation.
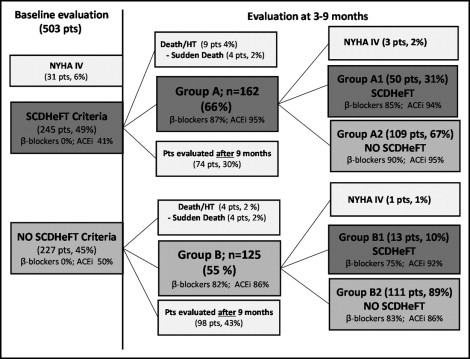
Of the 245 patients with baseline “SCD-HeFT criteria,” 9 (4%) had died (4 suddenly, 2%) before the second evaluation and 162 were fully re-evaluated 3 to 9 months (mean 5.4 ± 2) later (group A).
Of the 227 patients with a LVEF >0.35 and/or NYHA class I (group B), 4 (2%) had died (all suddenly) before the second evaluation and 125 were fully re-evaluated 3 to 9 months (mean 5.5 ± 2) later (group B). Thus, the study population (group A plus group B patients) included 287 patients. The clinical and laboratory findings of the study population at the first evaluation are summarized in Table 1 .
| Variable | Value |
|---|---|
| Age (years) | 43 ± 14 |
| Males | 215 (75%) |
| Body surface area (m 2 ) | 1.87 ± 0.23 |
| Duration of heart failure (months) | 11 ± 21 |
| Heart rate at rest (beats/min) | 79 ± 15 |
| Systolic blood pressure (mm Hg) | 125 ± 15 |
| New York Heart Association class | |
| I | 95 (33%) |
| II | 129 (45) |
| III | 63 (22%) |
| Electrocardiographic findings | |
| Sinus rhythm | 270 (94%) |
| Left bundle branch block | 95 (33%) |
| Echocardiographic findings | |
| Indexed left ventricular end-diastolic diameter (mm) | 37 ± 5 |
| Indexed left ventricular end-diastolic volume (ml) | 108 ± 38 |
| Right ventricular area shortening fraction | 0.45 ± 0.16 |
| Left ventricular ejection fraction | 0.29 ± 0.09 |
| Mitral regurgitation area at echocardiography >4 cm 2 (4-chamber view) | 115 (40%) |
| Restrictive filling pattern ⁎ | 95 (33%) |
| Holter findings (number of nonsustained ventricular tachycardia/24 hours) | 4 ± 25 |
| Exercise stress test (functional capacity [W]) | 104 ± 41 |
| Drug therapy | |
| Angiotensin-converting enzyme inhibitors/angiotensin receptor blocker | 132 (46%) |
| β Blockers | 0 (0%) |
| Digoxin | 195 (68%) |
| Diuretics | 164 (57%) |
| Amiodarone | 26 (9%) |
⁎ Restrictive filling pattern: mitral E wave deceleration time ≤120 ms or mitral E wave deceleration time <150 ms associated with E/A ratio ≥2.
In 172 patients (74 with baseline SCD-HeFT criteria and 98 with a LVEF >0.35 and/or NYHA class I at baseline), a second full evaluation was performed >9 months later and they were excluded from the analysis ( Figure 1 ). At the first evaluation, these patients, compared to the study population, showed a slightly greater LVEF (0.33 ± 0.09 vs 0.29 ±0.09; p <0.001), lower end-diastolic diameter (35.5 ± 5 vs 37.5; p = 0.003), and less frequent LV restrictive filling pattern (in 23% vs 33%; p = 0.041). No differences were found concerning the demographic and clinical features, symptoms or duration of heart failure. Long-term survival was not different comparing these patients and the study population (data not shown).
ACE inhibitors or angiotensin receptor blockers were given to 46% of the study population before our first evaluation and to 91% of patients at the second evaluation (reaching an enalapril-equivalent dose of 22 ± 13 mg/day). At the second evaluation, β blockers were tolerated in 85% of patients, reaching a mean carvedilol-equivalent dosage of 51 ± 26 mg/day.
In both group A and B patients, a significant improvement occurred in the LV systolic and diastolic function and LV dimensions ( Tables 2 and 3 ), as well as a reduction in the heart rate and nonsustained ventricular arrhythmias. The improvement was more evident in patients with baseline SCD-HeFT characteristics (group A; Table 2 ).
| Variable | First Evaluation | Second Evaluation | p Value |
|---|---|---|---|
| Age (years) | 46 ± 13 | ||
| Males | 120 (74%) | ||
| Duration of heart failure (months) | 14 ± 23 | ||
| Heart rate at rest (beats/min) | 81 ± 17 | 71 ± 13 | <0.001 |
| Systolic blood pressure (mm Hg) | 124 ± 16 | 127 ± 18 | 0.035 |
| New York Heart Association class | <0.001 | ||
| I | 0 (0%) | 65 (40%) | |
| II | 105 (65%) | 78 (48%) | |
| III | 57 (35%) | 16 (10%) | |
| IV | 0 (0%) | 3 (2%) | |
| I–II | 105 (65%) | 144 (89%) | <0.001 |
| Electrocardiographic findings | |||
| Sinus rhythm | 151 (93%) | 149 (92%) | 1 |
| Left bundle branch block | 70 (43%) | 58 (36%) | 0.007 |
| Echocardiographic findings | |||
| Indexed left ventricular end-diastolic Diameter (mm) | 38 ± 5 | 36 ± 6 | <0.001 |
| Indexed left ventricular end-diastolic volume (ml) | 119 ± 37 | 102 ± 35 | <0.001 |
| Right ventricular area shortening fraction | 0.44 ± 0.17 | 0.47 ± 0.14 | 0.053 |
| Left ventricular ejection fraction | 0.24 ± 0.06 | 0.35 ± 0.11 | <0.001 |
| Mitral regurgitation area at echocardiography >4 cm 2 (4-chamber view) | 86 (53%) | 52 (32%) | <0.001 |
| Restrictive filling pattern ⁎ | 70 (43%) | 26 (16%) | <0.001 |
| Holter findings (number of nonsustained ventricular tachycardia/24 hours) | 4 ± 17 | 1 ± 2 | <0.001 |
| Exercise stress test findings (functional capacity [W]) | 95 ± 33 | 101 ± 37 | 0.013 |
| Drug therapy | |||
| Angiotensin-converting enzyme inhibitors/angiotensin receptor blocker | 66 (41%) | 154 (95%) | <0.001 |
| β Blockers | 0 (0%) | 141 (87%) | <0.001 |
| Enalapril equivalent dosage (mg/day) | Not known | 27 ± 13 | // |
| Carvedilol equivalent dosage (mg/day) | 0 | 53 ± 28 | // |
| Digoxin | 146 (90%) | 146 (90%) | 1 |
| Diuretics | 123 (76%) | 125 (77%) | 1 |
| Amiodarone | 15 (9%) | 15 (9%) | 1 |
⁎ Restrictive filling pattern: mitral E wave deceleration time ≤120 ms or mitral E wave deceleration time <150 ms associated with E/A ratio ≥2.
| Variable | First Evaluation | Second Evaluation | p Value |
|---|---|---|---|
| Age (years) | 40 ± 14 | ||
| Males | 94 (75%) | ||
| Duration of heart failure (months) | 6 ± 16 | ||
| Heart rate at rest (beats/min) | 77 ± 12 | 69 ± 11 | <0.001 |
| Systolic blood pressure (mm Hg) | 127 ± 15 | 126 ± 16 | 0.523 |
| New York Heart Association class | 1 | ||
| I | 94 (75%) | 92 (74%) | |
| II | 24 (19%) | 28 (22%) | |
| III | 8 (6%) | 4 (3%) | |
| IV | 0 (0%) | 1 (1%) | |
| I–II | 118 (94%) | 120 (96%) | 0.727 |
| Electrocardiographic findings | |||
| Sinus rhythm | 120 (96%) | 116 (93%) | 0.375 |
| Left bundle branch block | 25 (20%) | 25 (20%) | 1 |
| Echocardiographic findings | |||
| Indexed left ventricular end-diastolic Diameter (mm) | 35 ± 5 | 33 ± 5 | <0.001 |
| Indexed left ventricular end-diastolic volume (ml) | 96 ± 36 | 85 ± 34 | <0.001 |
| Right ventricular area shortening fraction | 0.50 ± 0.14 | 0.49 ± 0.12 | 0.578 |
| Left ventricular ejection fraction | 0.36 ± 0.08 | 0.41 ± 0.11 | <0.001 |
| Mitral regurgitation area at echocardiography >4 cm 2 (4-chamber view) | 26 (21%) | 20 (16%) | 0.286 |
| Restrictive filling pattern ⁎ | 20 (16%) | 10 (8%) | 0.092 |
| Holter findings (number of nonsustained ventricular tachycardia/24 hours) | 6 ± 32 | 1 ± 7 | 0.008 |
| Exercise stress test findings (functional capacity [W]) | 121 ± 43 | 124 ± 39 | 0.326 |
| Drug therapy | |||
| Angiotensin-converting enzyme inhibitors/angiotensin receptor blocker | 63 (50%) | 108 (86%) | <0.001 |
| β Blockers | 0 (0%) | 103 (82%) | <0.001 |
| Enalapril equivalent dosage (mg/day) | Not known | 23 ± 13 | |
| Carvedilol equivalent dosage (mg/day) | 0 | 63 ± 29 | |
| Digoxin | 73 (58%) | 70 (56%) | 0.688 |
| Diuretics | 41 (33%) | 36 (29%) | 0.424 |
| Amiodarone | 11 (9%) | 11 (9%) | 1 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


