Chapter 18 High pressure and diving
 When diving in water the increased density of inhaled gases and immersion in water cause an increase in the work of breathing, which can impair gas exchange during exercise.
When diving in water the increased density of inhaled gases and immersion in water cause an increase in the work of breathing, which can impair gas exchange during exercise. Above about 4 atmospheres absolute pressure nitrogen has anaesthetic effects and divers must breathe helium, which also overcomes the problem of increased gas density.
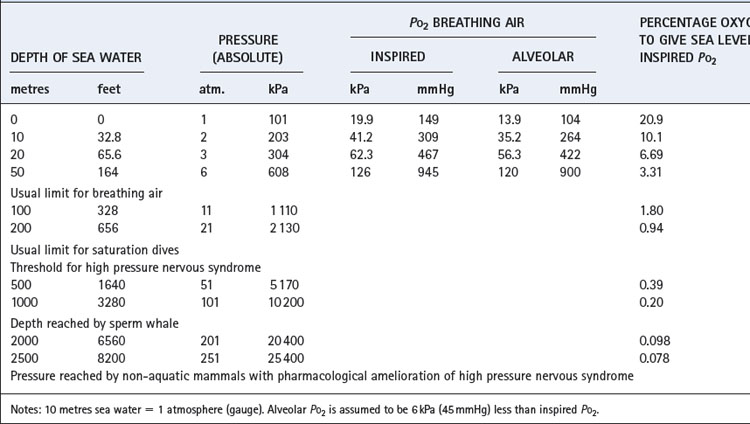
Above about 4 atmospheres absolute pressure nitrogen has anaesthetic effects and divers must breathe helium, which also overcomes the problem of increased gas density.In this field, as in others, we cannot escape from the multiplicity of units, and some of these are set out in Table 18.1. Note particularly that ‘atmosphere gauge’ is relative to ambient pressure. Thus 2 atmospheres absolute (ATA) equals 1 atmosphere gauge relative to sea level. Throughout this chapter atmospheres of pressure refer to absolute and not gauge.
Exchange of Oxygen and Carbon Dioxide1
Effect of Pressure on Alveolar Pco2 and Po2
Pressure has complicated and very important effects on Pco2 and Po2. The alveolar concentration of CO2 equals its rate of production divided by the alveolar ventilation (page 167). However, both gas volumes must be measured under the same conditions of temperature and pressure. Alveolar CO2 concentration at 10 ATA will be about one tenth of sea level values, i.e. 0.56% compared with 5.3% at sea level. When these concentrations are multiplied by pressure to give Pco2, values are similar at sea level and 10 atmospheres. Thus, as a rough approximation, alveolar CO2 concentration decreases inversely to the environmental pressure, but the Pco2 remains near its sea level value.
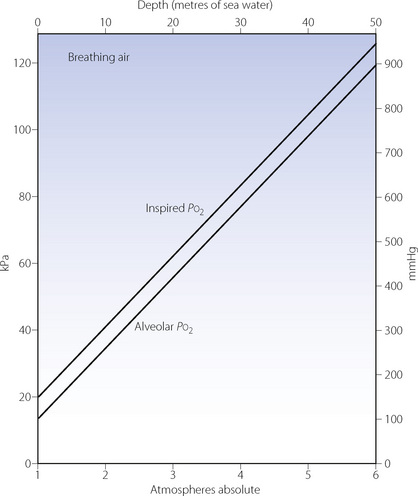
Effects on the Po2 are slightly more complicated but no less important. The difference between the inspired and alveolar oxygen concentrations equals the ratio of oxygen uptake to inspired alveolar ventilation. This fraction, like the alveolar CO2 concentration, decreases inversely with the increased pressure. However, the corresponding partial pressure will remain close to the sea level value, as does the alveolar Pco2. Therefore the difference between the inspired and alveolar Po2 will remain roughly constant, and the alveolar Po2, to a first approximation, increases by the same amount as the inspired Po2 (Figure 18.1). However, these considerations only take into account the direct effect of pressure on gas partial pressures. There are other, more subtle, effects on respiratory mechanics and gas exchange which must now be considered.
Effect on Mechanics of Breathing2,3,4
Gas density is increased in direct proportion to pressure. Thus air at 10 atmospheres has 10 times the density of air at sea level, which increases the resistance to turbulent gas flow (page 45) and limits the maximal breathing capacity (MBC) that can be achieved. In fact, it is usual to breathe a helium/oxygen mixture at pressures in excess of about 6 atmospheres because of nitrogen narcosis (see below). Helium has only one-seventh the density of air and so is easier to breathe. Furthermore, lower inspired oxygen concentrations are both permissible and indeed desirable as the pressure increases (Table 18.1). Therefore, at 15 atmospheres it would be reasonable to breathe a mixture of 98% helium and 2% oxygen. This would more than double the MBC that the diver could attain while breathing air at that pressure. Hydrogen has even lower density than helium, and has been used in gas mixtures for dives to more than 500 metres deep.5
The effect of immersion is additional to any change in the density of the respired gases. In open-tube snorkel breathing, the alveolar gas is close to normal atmospheric pressure but the trunk is exposed to a pressure depending on the depth of the subject, which is limited by the length of the snorkel tube. This is equivalent to a standing subatmospheric pressure applied to the mouth and it is difficult to inhale against a ‘negative’ pressure loading of more than about 5 kPa (50 cmH2O). This corresponds to a mean depth of immersion of only 50 cm, and it is therefore virtually impossible to use a snorkel tube at a depth of 1 metre. However, the normal length of a snorkel tube assures that the swimmer is barely more than awash, and so these problems should not arise.
These arrangements supply gas that is close to the hydrostatic pressure surrounding the trunk. However, the precise ‘static lung loading’ depends on the location of the pressure-controlling device in relation to the geometry of the chest. Minor differences result from the various postures that the diver may assume. Thus, if he is ‘head-up’ when using a valve at mouthpiece level, the pressure surrounding the trunk is higher than the airway pressure by a mean value of about 3 kPa (30 cmH2O). If he is ‘head-down’, airway pressure is greater than the pressure to which the trunk is exposed. The head-down position thus corresponds to positive pressure breathing and the head-up position to negative pressure breathing. The latter causes a reduction of functional residual capacity (FRC) of about 20–30% but breathing is considered to be easier head-up than head-down.1
Apart from these considerations, immersion has relatively little effect on respiratory function, and the additional respiratory work of moving extracorporeal water does not seem to add appreciably to the work of breathing.
Effect on Efficiency of Gas Exchange4
Dead space/tidal volume ratio in divers increases with greater depth.2,6 Changes are seen at relatively low pressures, for example, in one study dead space/tidal volume ratio increased from 37% at sea level to 42% at 2.8 ATA.2 During exercise at this pressure, values decreased to around 20%.
The best measure of the efficiency of oxygenation of the arterial blood is the alveolar/arterial Po2 gradient. Measurement of arterial blood gas tensions presents formidable technical difficulties at high pressures. However, studies at 2.8, 47 and 66 ATA have reported only small increases in alveolar/arterial Po2 gradient.2,6 Since it is customary to supply deep divers with an inspired oxygen tension of at least 0.5 ATA, arterial hypoxaemia is unlikely to occur either from hypoventilation or from maldistribution of pulmonary ventilation and perfusion in healthy subjects.6
The position as regards arterial Pco2 is less clear. Hypercapnia is a well-recognised complication of diving, and divers are known to have a blunted Pco2/ventilation response, though the cause of this is unknown.1 Hypercapnia in divers at rest is uncommon, but during exercise elevated end-tidal and arterial Pco2 levels are described. Arterial Pco2 during exercise at 2.8 ATA were around 5 kPa (37 mmHg),2 but at pressures of 47 and 66 ATA were in the range 6.2–8.3 kPa (47–62 mmHg).6 This is potentially hazardous because 9 kPa is approaching the level at which there may be some clouding of consciousness, and that is potentially dangerous at depth. High gas density at depth causing increased work of breathing is believed to be responsible for the inadequate ventilation during exercise.
Oxygen Consumption
The relationship between power output and oxygen consumption at pressures up to 66 ATA, whether under water or dry, is not significantly different from the relationship at normal pressure6 shown in Figure 15.1. Oxygen consumption is expressed under standard conditions of temperature and pressure, dry (STPD, see Appendix C) and therefore represents an absolute quantity of oxygen. However, this volume, when expressed at the diver’s environmental pressure, is inversely related to the pressure. Thus, an oxygen consumption of 1 l.min−1 (STPD) at a pressure of 10 atmospheres would be only 100 ml.min−1 when expressed at the pressure to which the diver is exposed. Similar considerations apply to carbon dioxide output.
The ventilatory requirement for a given oxygen consumption at increased pressure is also not greatly different from the normal relationship shown in Figure 15.5
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree