Fig. 6.1
Herpesvirus-1 ×7500 (Courtesy M. Selig)
6.4 Immunology
Herpesvirus types 1 and 2 can both infect the lung (Ramsey et al. 1982). HSV-1 is the most frequent cause of primary disease, whereas HSV-2 generally results from viremic spread. All herpesviruses are nuclear replicating, i.e., the viral DNA is transcribed to mRNA within the infected cell’s nucleus and the mRNA gene products promote the replication of the viral DNA. Infection is initiated when a viral particle contacts a cell with specific types of cell surface membrane receptors. Following binding of viral envelope glycoproteins to their receptors, the virion is internalized and dismantled, so that viral DNA can migrate to the cell nucleus where replication of viral DNA and transcription of viral genes occur.
During symptomatic infection, infected cells transcribe lytic viral genes. In some host cells, a small number of viral genes termed latency-associated transcripts (LAT) accumulate and allow the virus to persist in the host cell indefinitely. During latency, the infected host is asymptomatic, but reactivation of latent viruses can result in disease. In such case, the transcription of latency-associated LAT genes switches to that of lytic genes that lead to enhanced virus production and cell death. Symptoms and signs may include low-grade fever, headache, sore throat, and rash, as well as lymphadenopathy and reduced levels of circulating immune cells. Infection is long lived, as viral DNA persist in dormant states within sensory nerves and ganglia. HSV-1 reactivation begins in peripheral nerve cells, and viruses are transported via sensory nerve axons to mucosal surfaces, where they can produce vesicular eruptions.
6.5 Clinical Features
Young age, airway trauma, airway burns, prolonged mechanical ventilation, and immunosuppression are risk factors for developing herpetic pneumonia (Cherr et al. 2000; Sherry et al. 1988). Newborn infants are particularly at risk and may develop disseminated disease with pulmonary infiltrates and mortality rates (even following acyclovir therapy) approaching 30 % (Kimberlin 2004). In adults, HSV is isolated from secretions in ~30 % of mechanically ventilated patients, and evidence suggests that Herpesviruses may be an important factor in severe exacerbations of chronic obstructive pulmonary disease (Gu and Korteweg 2007). Dyspnea, cough, and hypoxemia are common in this setting. Acute Respiratory Distress Syndrome (ARDS) can develop with virulent herpetic infection, and bacterial and fungal superinfections are both common and can be fatal when they supervene. Herpesvirus can also complicate ARDS (Byers et al. 1996).
The chest radiographic appearances of Herpesvirus infection range from mucosal thickening of the pulmonary airways, multifocal areas of consolidation, and diffuse bilateral pulmonary infiltrates. Herpesvirus tracheobronchial infections tend to complicate labial and esophageal disease with virus spreading via the aspiration of oropharyngeal secretions (Corey and Spear 1986; Feldman and Stokes 1987). Intubated patients receiving chronic ventilatory support are at risk as a consequence of local mucosal barotrauma from inflated endotracheal tubes.
6.6 Pathological Changes
The respiratory mucosa is the primary target, and Herpesvirus characteristically produces ulceration and extensive necrosis (Nash and Foley 1970). The ballooning of infected cells, cell karyorrhexis, and piling up of the infected cells suggest the diagnosis. There may be a prominent neutrophilic response that mimics a pyogenic bacterial infection (Fig. 6.2). Immunosuppressed patients with Herpesvirus viremia may develop miliary foci of pulmonary hemorrhagic necrosis with prominent alveolar fibrin exudates (Graham 1983) (Fig. 6.3). The foci of infection may be paucicellular although scattered neutrophilic exudates are frequently present. Diagnostic cytopathic changes may be difficult to identify but will usually be visualized after detailed examination of the involved foci.
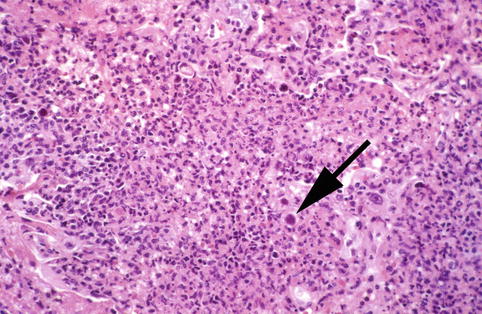
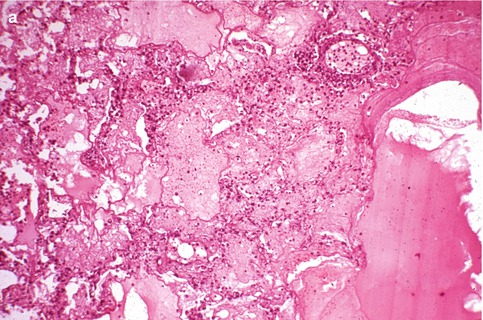
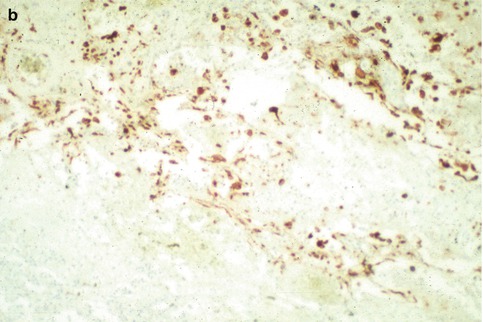

Fig. 6.2
Neutrophilic exudates shows Herpesvirus-1 infected cell (arrow)


Fig. 6.3
(a) Hemorrhagic necrotizing pneumonia in immunosuppressed patient with Herpesvirus-1 viremia. (b) Multiple infected cells immunostaining for Herpesvirus-1
6.7 Diagnosis
The diagnosis of airway disease can be established by viral isolation from respiratory secretions, bronchoalveolar lavage fluids, or mucosal biopsies of ulcerated sites (Fig. 6.4). As immunohistochemistry shows antigenic overlap between HSV-1 and HSV-2 infections, PCR methods may be required for accurate speciation. Diagnostic cytopathic changes include the presence of either type A or type B Cowdry nuclear inclusions, molding of adjacent cells, and multikaryon formation (Fig. 6.5). When there is extensive necrosis, immunohistochemistry for herpes viral antigen will often demonstrate intense background staining. While this suggests the diagnosis, it can potentially obscure it (Strickler et al. 1990) (Fig. 6.6b). The examination of paraffin-embedded tissues by electron microscopy can help to confirm the diagnosis.
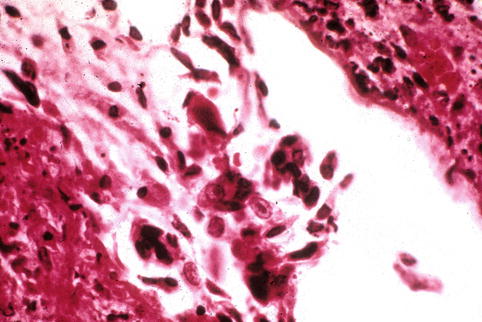
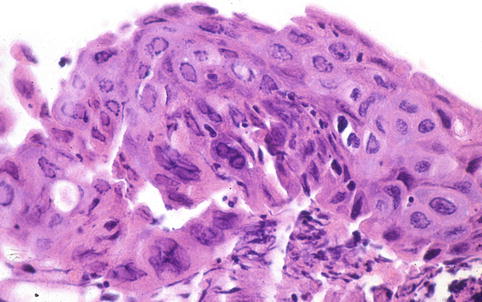
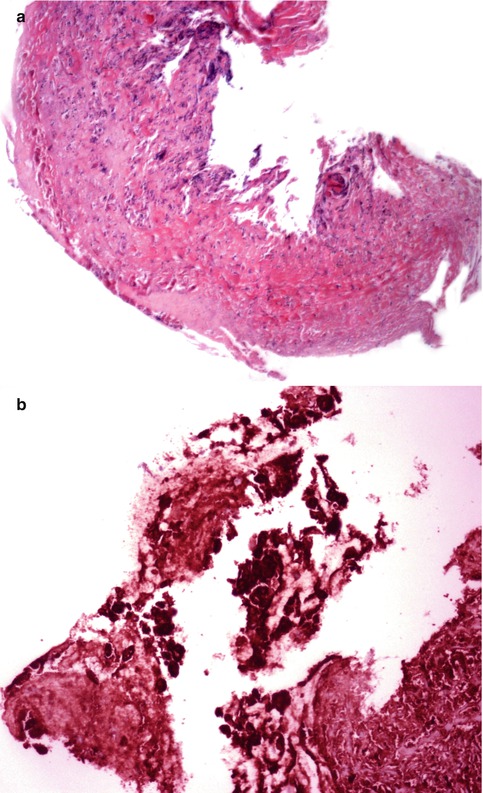

Fig. 6.4
Herpetic inclusions in squamous respiratory epithelium of a chronically intubated patient

Fig. 6.5
Polykaryons with nuclear inclusions in herpetic pneumonia

Fig. 6.6
(a) Ulcerated tracheal lesion showing (b) intense immunostaining for Herpesvirus-1. The diagnosis was confirmed by ultrastructural examination demonstrating diagnostic virions
6.8 Differential Diagnosis
Necrotizing hemorrhagic pneumonias due to Herpesviruses are similar histopathologically to those associated with varicella zoster, adenovirus, and cytomegalovirus (CMV). Helpful in making the distinction are the location and character of the viral inclusions. For example, smudge cells are strongly suspicious of adenovirus while cytomegaly is more apt to be seen in CMV infection. Immunohistochemistry plays an important role and can help to refine the diagnosis.
6.9 Treatment
Therapy is based primarily on the usage of antiviral agents such as acyclovir, valacyclovir, and famciclovir. Drug-resistant strains may respond to fosecarnet. Fosecarnet has been found effective in some acyclovir-resistant patients. Supportive therapy, hydration, and treatment of associated bacterial infection are indicated in selected patients.
6.10 Clinicopathologic Capsule
Herpesviruses can infect the lung as well as the mucosa of the tracheobronchial tree. Young age, airway trauma, prolonged mechanical ventilation, and immunosuppression are risk factors for development of herpetic infections. Biopsy samples from the respiratory mucosa showing ballooning of infected cells, cell karyorrhexis, and piling up of infected cells suggest the diagnosis. The diagnosis can be confirmed with appropriate immunohistochemical stains or by identification of Cowdry type A or B in infected cells. A rare and distinctive type of malignant lymphoma occurs in patients infected with Human Herpesvirus-8. This lymphoma has equally distinctive molecular features (mutations in the 5″ noncoding regions of bcl6) that help to make the diagnosis.
6.11 Variants
6.11.1 Human Herpesvirus-6
Studies of lung tissues and bronchoalveolar lavage specimens from patients with pneumonia have led some investigators to propose Human Herpesvirus 6 (HHV-6) as a clinical cause of pneumonia (Cone et al. 1994). Cases should be referred to as “HHV-6-associated” pneumonia. Both mild and severe cases of pneumonia and bronchiolitis obliterans organizing pneumonia (BOOP) have been reported for HHV-6 infection occurring in immunosuppressed individuals with HIV or following bone marrow transplantation. No systematic evaluation of treatment regimens is currently available, and controlled prospective studies are required to confirm HHV-6 as a primary pulmonary pathogen.
6.11.2 Human Herpesvirus-8
In addition to its role in Kaposi’s sarcoma and multicentric Castleman’s disease, HHV-8 has been suggested as a cause of interstitial pneumonitis. Primary effusion lymphoma, previously called body-cavity-based lymphoma, is a rare, distinctive type of HHV8+ diffuse large B-cell lymphoma characterized by lymphomatous effusions involving pleural, pericardial, or peritoneal cavities unaccompanied by a solid mass (Ansari et al. 1996; Cesarman et al. 1995; Said and Cesarman 2008). Here we discuss only primary effusion lymphoma.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


