Fig. 13.1
Henry Cavendish (1731–1810). This sketch was made surreptitiously by William Alexander. From [16]
Cavendish was a very eminent scientist but not a major figure in physiology. However his work is of considerable interest. He was the first person to prepare and understand the nature of hydrogen. He made this by adding acid to metal filings. The gas had previously been produced by Robert Boyle (1627–1691) but he confused it with ordinary air. Cavendish also worked on carbon dioxide, or “fixed air” as it was called. This had previously been prepared by Joseph Black (1728–1799) but his description was brief and Cavendish elucidated its properties. Another important area of research was the properties of water. Joseph Priestley had previously observed that when a mixture of inflammable and common air was exploded with an electric spark, the container “became dewey”. Cavendish went on to study the properties of water in detail. Another interest was the composition of atmospheric air which he was the first to accurately determine. He also worked on nitric acid but all his chemistry research was interpreted in terms of the phlogiston theory. This stated that a fire-like element was released during combustion. Having said this, he was one of the first people outside France to recognize the shortcomings of this theory after the crucial studies by Antoine Lavoisier (1747–1794).
Cavendish worked in other areas including experiments on heat. Here he expanded on the work of Black on latent heat. However the major contribution of Cavendish to science outside chemistry was in the field of gravitational attraction. His major project now known as “the Cavendish experiment” was a meticulous measurement of the gravitational attraction between lead balls in his laboratory carried out with extreme precision [6]. The results allowed him to calculate the average density of the world which he gave as 5.45 times that of water. He referred to this experiment as “weighing the world”. As a result, many physicists credit Cavendish with the first accurate measurement of Newton’s gravitational constant.
There are three major biographies of Cavendish. The most recent [9] is over 800 pages long and is exhaustive. This is an extension of an earlier book [8] and contains many letters to and from Cavendish. An earlier biography [1] concentrates on his published papers, and a much earlier volume [16] deals extensively with the controversy between Cavendish and Watt on the discovery of the composition of water. A collection of the Cavendish papers is available [10] and several are reproduced on the Internet. Partington [12] has a good discussion of the chemical studies.
13.2 Hydrogen
One of the first publications by Cavendish was titled “Three papers, containing Experiments on factitious Air” [2] and it appeared in the journal Philosophical Transactions in 1766. The purpose of this journal was to “register”, that is print, communications that had been given to the Royal Society. The three papers were presented to the Society on May 29, November 6 and November 13, 1766. Philosophical Transactions dealt with all areas of science and the discussions at the Society meetings covered a bewildering array of topics. For example the first paper on inflammable air was preceded by two on a recent solar eclipse, another on the double horns of a rhinoceros, and a third on a very large hernia. Following Cavendish’s presentations there was one on men who were 8 ft. tall or more in Patagonia, another on locked jaw apparently cured by electricity, and a third on a swarm of gnats in Oxford. This disconcerting collection emphasizes the lively, effervescent intellectual activity in the Royal Society at the time.
By factitious air Cavendish meant “any kind of air which is contained in other bodies in an unelastic state, and is produced from thence by art” [2]. The term had originally been introduced by Boyle. The first paper was on “Inflammable air” which we know today as hydrogen. Other investigators such as Boyle had previously prepared this gas but had not realized what it was. The detailed studies of Cavendish allowed him to be credited as the first person to recognize its true nature.
Cavendish prepared the gas by the action of acids on various metals. The two acids that he used were spirit of salt (hydrochloric acid) and dilute oil of vitriol (sulfuric acid). He studied three metals, zinc, iron and tin. Various types of apparatus were used in his experiments and these are illustrated in his publication (Fig. 13.2). Cavendish described inflammable air as “permanently elastic” which was a term used to describe all gases at the time. He showed that the volume remained constant in spite of the gas being exposed to water or alkalis. In fact it was so insoluble in water that there was no measurable absorption over a period of several weeks. Finally he described that it was explosive when it was mixed with common air and exposed to a spark, and he noted that this behavior had been described by others.
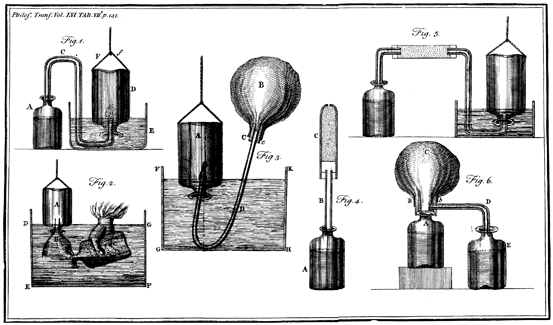
Fig. 13.2
Apparatus used by Cavendish in his studies of hydrogen, carbon dioxide, atmospheric air, and water. From [2]
An important determination was the density of the new gas. It was easy to measure a given volume by water displacement using the equipment shown in Fig. 13.2. However measuring the weight of a given volume of gas was much more challenging. This was done by placing a known volume of the gas in a bladder as shown in the third image of Fig. 13.2 and weighing it using a sensitive analytical balance. One of Cavendish’s balances that still exists today is shown in Fig. 13.3. Other scientists at the time, for example Black, had also developed very accurate balances. Cavendish measured the density of several samples of hydrogen and compared the density with that of common air and water. He reported that the mean density was “8700 times lighter than water”. This meant that it was much lighter than air which had “its specific gravity… 7840 times less than that of water”. The paper read by Cavendish on May 29 describing his work on inflammable air was warmly received, and the secretary of the Society, Henry Oldenburg, wrote in the Society’s Journal Book that “It is impossible to do Justice to the Experiments under the title “Of inflammable air” without citing them wholly” [14].

Fig. 13.3
Chemical balance used by Cavendish. This was described as “of rude exterior but singular perfection”. It is now in the Royal Institution in London. (By permission of the Royal Institution)
13.3 Carbon Dioxide
The second of the three papers was read by Cavendish on November 6, 1766 and was about his work on “Fixed air”. We now know this as carbon dioxide and it had previously been produced by Joseph Black although his description was brief. Cavendish produced fixed air by the same methods as used by Black, that is by adding acid to alkalis such as calcium carbonate or magnesium carbonate, or heating these substances, that is what is known as calcination. Cavendish explored the properties of this gas using the same techniques as he had for inflammable air. He reported that it was permanently elastic like inflammable air but in other respects it was different. First it was soluble in water and for that reason he collected it over mercury. He also found that it was not flammable and he reported its density as 511 times lighter than water, or 1.57 times heavier than common air.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


