Hemothorax
Hemothorax is the presence of a significant amount of blood in the pleural space. Most hemothoraces result from penetrating or nonpenetrating chest trauma. An occasional hemothorax results from iatrogenic manipulation such as the placement of central venous catheters percutaneously by the subclavian or internal jugular route or from translumbar aortography. On rare occasions, a hemothorax results from a medical condition such as pulmonary embolism or rupture of an aortic aneurysm.
Blood may enter the pleural space from injury to the chest wall, diaphragm, lung, or mediastinum. Blood entering the pleural space coagulates rapidly. Presumably as a result of physical agitation produced by movement of the heart and the lungs, the clot may be defibrinated. Loculation occurs early in the course of hemothorax, as with empyema.
When a diagnostic thoracentesis in a medical patient reveals pleural fluid that appears to be pure blood, a hematocrit should always be obtained on the pleural fluid. Frequently, although the pleural fluid appears to be blood, the hematocrit on the pleural fluid is less than 5%. A hemothorax should be considered to be present only when the pleural fluid hematocrit is equal to or greater than 50% of the peripheral blood hematocrit. If a measured hematocrit is not available, a rough estimate of the hematocrit can be obtained by dividing the pleural fluid red blood cell (RBC) count by 100,000. For example, a pleural fluid RBC count of 1,000,000 equates with a pleural fluid hematocrit of 10%.
TRAUMATIC HEMOTHORAX
Traumatic hemothoraces are a frequent occurrence, particularly in centers that treat victims of trauma. In one Houston hospital, more than 300 patients with hemothorax due to penetrating trauma were seen in a 1-year period (1). The relative incidence of hemothorax due to penetrating and blunt thoracic trauma depends on whether the medical center cares primarily for victims of automobile accidents or of stab and gunshot wounds.
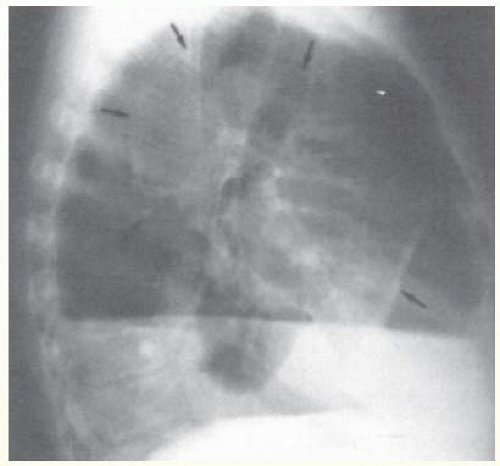
There is a high incidence of hemothorax with blunt trauma. In a retrospective analysis of 515 cases of blunt chest trauma, 193 patients (37%) had hemothoraces (2). In patients with rib fractures, hemothorax is more common if the fracture is displaced (3). Pneumothorax occurring concomitantly with hemothorax is common whether the trauma is blunt or penetrating (Fig. 25.1).
In a series of 114 patients with hemothorax secondary to blunt trauma, 71 (62%) also had pneumothorax (4). In another series of 373 patients with hemothorax secondary to penetrating trauma, 307 (83%) also had pneumothorax (1).
In a series of 114 patients with hemothorax secondary to blunt trauma, 71 (62%) also had pneumothorax (4). In another series of 373 patients with hemothorax secondary to penetrating trauma, 307 (83%) also had pneumothorax (1).
Diagnosis
The diagnosis of a traumatic hemothorax should be suspected in any patient with penetrating or nonpenetrating trauma to the chest. The diagnosis is usually established by the demonstration of a pleural effusion with a chest radiograph or with ultrasound. As an initial screening test, surgeon-performed ultrasonography appears to be as sensitive as a supine chest radiograph in detecting hemothorax. In one study of 360 patients, 39 of 40 effusions were detected by ultrasound and 37 were detected by chest radiograph. The performance time for ultrasonography was significantly faster than that for chest radiography (1.3 vs. 14.2 minutes) (5). In some patients, a hemothorax becomes apparent only after some delay. In one series of 167 hemothoraces from Toronto due to blunt trauma, the initial chest radiograph revealed no hemothorax in 7 patients, but a hemothorax was subsequently diagnosed 22 hours to 16 days later in these 7 (4.2%) (6). All seven patients had multiple rib fractures, which were displaced in five (6).
A case can be made for obtaining chest computed tomography (CT) scans in all patients with severe chest injuries. Trupka et al. (7) obtained supine chest radiographs and chest CT scans in 103 patients with severe chest injuries and reported that the chest radiograph missed hemothoraces in 21 patients, lung contusion in 33 patients, and pneumothorax in 27 patients. In a second study of 93 patients with blunt trauma to the chest, 25 had normal supine chest radiographs and the chest CT scan showed multiple injuries in 13 patients including two aortic lacerations, three hemothoraces, and one pericardial effusion (8).
Treatment
The treatment of choice for patients with traumatic hemothorax is the immediate insertion of a chest tube. Obviously, if there is only a very small hemothorax, tube thoracostomy is not necessary. An occult hemothorax is one that is seen on CT scan but not apparent on the supine chest radiograph. Tube thoracostomy is not necessary for most patients with occult hemothorax (9). In one study, tube thoracostomy was performed if either diaphragmatic dome was obscured or if the fluid was more than 2 cm in thickness on the lateral decubitus radiograph (10). Most patients need tube thoracostomy for a relatively short period. In one study of 1,845 patients from South Africa, chest tubes were checked every 6 hours and were removed when there was no air leak and there was less than 50 mL drainage in the previous 6 hours (10). With this protocol, the average drainage time was 27.1 hours and 82% of the patients were discharged in less than 48 hours (10). Chest tubes should be removed as soon as they stop draining or cease to function because they can serve as conduits for pleural infection.
In the past, it was believed by some that the insertion of a chest tube would decrease pleural pressure and would thereby augment the pleural bleeding. If the bleeding originates from lacerated pleura, however, apposition of the pleural surfaces will produce a tamponade and will stop the bleeding (11). If the bleeding is from larger vessels, the slight decrease in the pleural pressure with a chest tube is insignificant in comparison to the transvascular pressure (11). The advantages of the immediate institution of tube thoracostomy are as follows: (a) it allows more complete evacuation of the blood from the pleural space; (b) it stops the bleeding if the bleeding is from pleural lacerations; (c) it allows one to quantitate easily the amount of continued bleeding; (d) it may decrease the incidence of subsequent empyema because blood is a good culture medium (12); (e) the blood drained from the pleural space may be autotransfused (1); and (f) the rapid evacuation of pleural blood decreases the incidence of subsequent fibrothorax (13).
Large-bore chest tubes (size 24 to 36 F) should be inserted in patients with hemothorax because the blood frequently clots (14). Beall et al. (12) recommend inserting the chest tube high (fourth or fifth intercostal space) in the midaxillary line because the diaphragm may be elevated by the trauma. Immediate thoracotomy or thoracoscopy is indicated for suspected cardiac tamponade, vascular injury, pleural contamination, debridement of devitalized tissue, sucking chest wounds, or major bronchial air leaks (15). Vascular injury is suggested if the initial chest tube output is more than 1,500 mL.
Continued pleural hemorrhage is another indication for immediate thoracotomy or video-assisted thoracic surgery (VATS). There is no precise criterion for the amount of pleural bleeding that should serve as an indication for surgery, because each case must be considered individually (11); however, if the
bleeding is at a rate of more than 200 mL/hour and shows no signs of slowing, thoracotomy or VATS should be seriously considered. Approximately 10% to 20% of patients with hemothorax require thoracotomy or VATS (1,3,4,10,12).
bleeding is at a rate of more than 200 mL/hour and shows no signs of slowing, thoracotomy or VATS should be seriously considered. Approximately 10% to 20% of patients with hemothorax require thoracotomy or VATS (1,3,4,10,12).
One must ensure that the bleeding is not from a misplaced central venous catheter (16,17). Mattox and Fisher (16) reported seven patients with a traumatic hemothorax in whom continued bleeding originated from a misplaced central venous catheter. This diagnosis is readily established by examining the appearance of the pleural drainage when the character of the infusion fluid is changed. If blood is obtained when fluid is withdrawn from the central catheter, the catheter may still be misplaced in the pleural space (18).
VATS is replacing thoracotomy in some patients with traumatic hemothorax who otherwise would have been subjected to thoracotomy. Thoracotomy rather than VATS should be performed if there is exsanguinating hemorrhage through the chest tubes (19,20). However, in the hemodynamically stable patient with persistent bleeding VATS is very effective (21). Villavicencio et al. (22) in a literature review found that VATS was effective in controlling the bleeding in 33 of 40 (82%) such cases. VATS was effective in controlling the bleeding when the bleeding arose from intercostal vessels and lung lacerations. Manlulu et al. (20) reported that VATS was the definitive procedure in all 19 patients in a single institution on whom it was attempted. Indications for VATS in this study included continued hemorrhage in six, retained hemothorax in six, and suspected diaphragmatic injury in three (20).
An alternative approach to the patient with persistent bleeding is to perform a contrast-enhanced CT scan and then perform transcatheter arterial embolization in patients who exhibit contrast extravasation. Hagiwara et al. (23) used this approach in 6 patients who were draining >200 ml/hour from their chest tube and reported that 5 had contrast extravasation and the bleeding was controlled in all five via transcatheter arterial embolization.
The efficacy of prophylactic antibiotics for the prevention of empyema in patients with tube thoracostomy for hemothorax is unclear. In an early study, Brunner et al. (24) randomly allowed 90 such patients to receive cefazolin or nothing immediately before and then every 6 hours until tube removal. They reported that there were six empyemas and three pneumonias in the control group, but only one pneumonia and no empyema in the group that received the antibiotic (24). However, in a more recent study, Maxwell et al. (25), in a randomized, double-blind study, gave cefazolin for 24 hours, for the duration of tube thoracostomy or placebo to 224 patients. The use of antibiotics did not significantly affect the incidence of empyema or pneumonia (25). However, only 1.3% of the patients who received antibiotics developed empyema while 5.6% of the patients receiving no antibiotics developed empyema. A longer duration of tube thoracostomy and a higher thoracic trauma score were associated with a higher incidence of empyema (25).
It appears that prehospital autotransfusion has a role in the management of life-threatening hemothorax. Barriot et al. (26) developed a system by which autotransfusions could be administered in ambulances. The system consists of a 28-to-30 F plastic chest tube and an autotransfusion device. The latter is basically a 750-mL bag with filters. The blood drains by gravity into the collection bag and is then reinfused without anticoagulation into a central line. They reported the use of their system on 18 patients in Paris with life-threatening traumatic hemothorax. During transfer to the hospital, the patients received 4.1 ± 0.6 L of autotransfused blood, without anticoagulation. Thirteen of the 18 patients (72%) survived, and there were no complications. They believed that the 13 patients would have died had it not been for the autotransfusions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree