Chapter 20 Hemodynamic Instability and Resuscitation
Hemodynamic instability refers to abnormalities of heart rate, blood pressure, filling pressures, or cardiac output that if uncorrected result in organ dysfunction. Hemodynamic instability is common in the intensive care unit (ICU) and encompasses a range of clinical states, from cardiac arrest to subtle tissue hypoperfusion. When hemodynamic instability is obvious and severe, timely intervention may be lifesaving. Equally important is the early recognition and treatment of slowly evolving or subclinical tissue hypoperfusion which, untreated, may progress to organ failure.
This chapter is divided into three sections: (1) postoperative hypertension; (2) hypotension and low cardiac output; (3) cardiac arrest and near cardiac arrest. Relevant physiology and pharmacology are discussed in Chapters 1 and Chapter 3, respectively; echocardiography is reviewed in Chapter 7; and hemodynamic monitoring is discussed in Chapter 8.
POSTOPERATIVE HYPERTENSION
Hypertension occurs because of increased systemic vascular resistance, increased cardiac output, or both (see Eq. 1-5). With increased systemic resistance, diastolic, systolic, and mean arterial pressures (MAP) are all increased. With increased stroke volume, diastolic pressure is usually normal or low, and pulse pressure is high. Postoperative hypertension has a number of causes (Table 20-1), but in most cases the primary problem is increased systemic vascular resistance. Surgical stress, hypothermia, patient anxiety, and inadequate analgesia lead to activation of the sympathetic nervous and renin-angiotensin-aldosterone systems. Essential hypertension is common and routine antihypertensive medications may have been withheld during the perioperative period.
Table 20-1 Etiology of Hypertension Following Cardiac Surgery
| Common Causes of Hypertension Following Cardiac Surgery |
| Pain |
| Anxiety (including paralysis combined with inadequate sedation) |
| Withdrawal of usual oral antihypertensive treatment (particularly β blockers) |
| Inappropriate vasopressor therapy |
| Hypervolemia |
| Hypothermia |
| Shivering |
| Patient-ventilator dysynchrony |
| Poorly controlled essential hypertension |
| Uncommon Causes of Hypertension Associated With Cardiac Surgery |
| Myocardial ischemia or infarction |
| Acute left ventricular failure |
| Drug treatment (corticosteroids, cyclosporine) |
| Coarctation of the aorta |
| Aortic dissection |
| Intracranial catastrophe |
| Uncommon Causes of Hypertension not Associated With Cardiac Surgery |
| Renal disease (including renal artery stenosis, end-stage renal disease, glomerulonephritis, etc.) |
| Endocrine dysfunction (including primary hyperaldosteronism, Cushing syndrome, pheochromocytoma, renin-producing tumor) |
| Toxemia of pregnancy |
| Arteritis |
Before antihypertensive drugs are given, the common causes of postoperative hypertension (see Table 20-1) should be considered and corrected. Clinicians must also assess the patient’s intravascular volume status and cardiac output (see subsequent discussion).
For intravenous antihypertensive therapy, vasodilators such as nitroglycerin, nicardipine, and nitroprusside are an attractive first choice because in patients with elevated systemic vascular resistance, they maintain or augment cardiac output. However, nitroglycerin may be ineffective in treating severe hypertension, and nitroprusside can cause marked hypotension, reflex tachycardia, and hypoxemia. Nesiritide may be considered when circulating volume is increased and filling pressures are high. If cardiac output is low, an inodilating drug such as milrinone is a good choice. Fluid loading may have to accompany vasodilating and inodilating drugs.
HYPOTENSION AND LOW CARDIAC OUTPUT
Recognizing and Treating Hemodynamic Instability
Hypotension
There are limited data concerning what constitutes an ideal blood pressure in critically ill patients. In one study of patients with septic shock, increasing MAP from 65 to 85 mmHg with norepinephrine did not improve markers of tissue perfusion or renal function.1 In the absence of similar studies in cardiac surgical patients, a target MAP of more than 65 mmHg is a reasonable goal for most patients. Suggested blood pressure targets in different groups of cardiac surgery patients are listed in Table 20-2.
Table 20-2 Blood Pressure Targets in the First 48 Hours Following Cardiac Surgery
| Normal (MAP >65 mmHg) |
| Default blood pressure goal |
| High (MAP >75-85 mmHg) |
| Age >75 years |
| Multiple arterial grafts |
| Preoperative or evolving renal impairment |
| Poorly controlled hypertension |
| History of ischemic stroke |
| New neurologic deficit postoperatively |
| Significant uncorrected carotid stenoses |
| Low (MAP >55-60 mmHg) |
| Age <50 years with no history of hypertension |
| High bleeding risk |
| Low blood pressure preoperatively with normal renal function |
| Valve surgery for chronically regurgitant valve lesions |
MAP, mean arterial pressure.
Low Cardiac Output and Tissue Hypoperfusion
Unlike blood pressure, cardiac output is not routinely measured in all patients, and clinicians often rely on clinical and biochemical markers of tissue hypoperfusion (Table 20-3). Unfortunately, clinical assessment of cardiac output is unreliable in the first few hours after cardiac surgery.2 Cool peripheries are normal findings. Polyuria is common, even with evolving renal dysfunction. Tachycardia may be absent due to the inhibitory effects of cardiopulmonary bypass (CPB) and surgery on the cardiac conducting system. Lactic acidosis is suggestive of tissue hypoperfusion, but other causes (e.g., β2-agonist drugs; see Table 31-3) may be responsible. Not uncommonly, low cardiac output occurs in the absence of lactic acidosis. A useful screening tool for low cardiac output is the oxygen saturation of blood drawn from the proximal port of a central venous catheter (i.e., from the superior vena cava; SSVCO2), which provides a close approximation of a true mixed venous oxygen saturation SVO2.3 Values above 65% are reassuring; values below 55% warrant further investigation. Other findings that demand further investigations include escalating inotropic support, a central venous pressure (CVP) above 15 mmHg, and unexplained metabolic acidosis (lactate >5 mmol/l, base deficit >6).
Table 20-3 Clinical and Biochemical Signs Consistent with Inadequate Cardiac Output
| Clinical |
| Cool, clammy peripheries |
| Sweating |
| Central hyperthermia |
| Oliguria with concentrated urine |
| Sinus tachycardia and atrial fibrillation |
| Narrow pulse pressure |
| Biochemical |
| Metabolic acidosis |
| Elevated lactate |
| Hyperkalemia |
| Low SVO2 or SSVCO2 |
SSVCO2, superior vena cava oxygen saturation; SVO2, mixed venous oxygen saturation.
If low cardiac output is suspected—and the cause is not readily apparent on the basis of routine clinical assessment—cardiac output should be measured. Numerous devices may be used (see Chapter 8), but in cardiac surgery units, the pulmonary artery catheter (PAC) is the device most commonly employed. A PAC also allows measurement of pulmonary arterial pressure, pulmonary artery wedge pressure (PAWP), and SVO2. As with blood pressure, the normal value for cardiac output is ill defined. By convention, a lower limit of 2.2 l/min/m2 is widely used; however, many “well” cardiac surgery patients have values below this figure.2 Also, the appropriate cardiac output depends on the patient’s metabolic state. Thus, in a patient with a marked systemic inflammatory response to CPB, a cardiac output of 4 l/min/m2 may be appropriate, whereas in a sedated, mildly hypothermic patient, a value of 2 l/min/m2 may be satisfactory. As a simple guide, if filling pressures are normal (PAWP <15 mmHg, CVP <12 mmHg), and venous oxygen saturation is satisfactory (>65%), a cardiac output as low as 1.8 l/min/m2 is probably acceptable.
Diagnosis and Treatment
Causes of hemodynamic instability in the early postoperative period are listed in Table 20-4. A stepwise approach to the diagnosis and initial treatment of hemodynamic instability is provided in Table 20-5. Important diagnostic clues can be obtained from the patient’s history and intraoperative course. The operation notes, angiograms, and echocardiograms should be reviewed. Examination should focus on the cardiovascular system, in particular the presence of any new murmurs. Specific diagnoses may be suggested on the basis of the electrocardiogram (ECG), CVP, and arterial pressure waveforms (see Chapter 8). If the ECG trace is abnormal, 12-lead and atrial ECGs should be obtained. Respiratory problems can cause hemodynamic instability, and a careful respiratory system examination, including checking the ventilator circuit and settings, should be performed. Chest drain bottles should be inspected for blood loss and bubbling. Important trends may be identified on the 24-hour ICU chart. Further blood tests, such as arterial blood gases, complete blood count, coagulation status, and troponin may be indicated, depending on the circumstances. Chest radiographs should be reviewed.
Table 20-4 Causes of Hemodynamic Instability in the Early Period Following Cardiac Surgery
| Common | Uncommon |
|---|---|
| Patient-ventilator dysynchrony | Severe mitral regurgitation |
| Hypovolemia | Other valvular pathology |
| Low systemic vascular resistance | Dynamic LVOT obstruction |
| Left ventricular systolic dysfunction | Dynamic lung hyperinflation |
| Left ventricular diastolic dysfunction | Tension pneumothorax |
| Right ventricular dysfunction | Massive hemothorax |
| Pericardial compression (tamponade) | |
| Rhythm disturbance |
LVOT, left ventricular outflow tract.
Table 20-5 A Stepwise Approach to the Diagnosis and Initial Treatment of the Hemodynamically Unstable Postoperative Cardiac Patient
| Step 1. Confirm the Presence of Hemodynamic Instability. |
| Check the level and zero all transducers. Relevel and rezero all pressure transducers if necessary. |
| Check all infusion pumps and the integrity of all infusion lines. |
| Step 2. Does the Patient Have an Immediately Life-threatening Problem (i.e., MAP < 50 mmHg)? |
| If so, go to Fig. 20-3. |
| Step 3. Clinically Assess the Patient (Steps 3 and 4 Should Occur Simultaneously). |
| Perform a targeted physical exam, concentrating on the cardiac and respiratory systems, the ventilator, and the chest drains. |
| Review the 24-hour chart. |
| Obtain relevant investigations: blood gases, SSVCO2, ECG (atrial and 12-lead), and chest radiograph. |
| Review the old notes and the intraoperative course. |
| Inform the surgeon. |
| Step 4. Consider the Following Interventions. |
| Paralyze and sedate the patient and ventilate with 100% oxygen. If indicated, disconnect the patient from the ventilator and hand ventilate with a manual resuscitator. |
| Pace the heart at 90 beats/min using DDD or DOO mode at maximum output (see Chapter 21). |
| Administer a fluid challenge of 500 ml of a crystalloid (e.g., normal saline or Plasma-Lyte). |
| Commence or increase inotropic support. |
| Step 5. If the Diagnosis Remains Uncertain, Insert a PAC and/or Perform an Echocardiogram. |
| If there is clinical suspicion of low or high cardiac output, a PAC should be inserted. |
| If a PAC is in place and cardiac output is low, an echocardiogram should be performed. |
| If the patient remains hypotensive and the cause is unclear, an echocardiogram should be performed. |
| If a specific diagnosis (e.g., tamponade) is suspected clinically, an echocardiogram should be performed. |
ECG, electrocardiogram; MAP, mean arterial pressure; PAC, pulmonary artery catheter.
If the cause of hypotension or low cardiac output is not rapidly apparent, an echocardiogram should be performed. Pulmonary artery catheterization and echocardiography are complementary techniques: a PAC is preferred for measuring cardiac output, whereas an echocardiogram is preferred for diagnosing the cause of low cardiac output and hypotension. In the ICU, transesophageal echocardiography (TEE) offers significant advantages over transthoracic echocardiography (TTE) (see Chapter 7).
Causes of Early Hemodynamic Instability
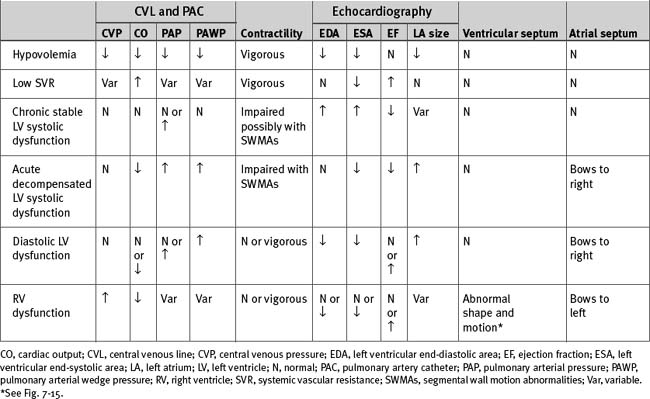
The causes of hemodynamic instability that occur within the first 48 hours after cardiac surgery are listed in Table 20-4; important hemodynamic and echocardiographic findings are summarized in Table 20-6.
Hypovolemia
Hypovolemia is a reduction in circulating blood volume such that left ventricular preload is inadequate to optimize cardiac output (see Chapter 1). Preload is usually equated with left ventricular end-diastolic volume (LVEDV), but surrogates such as left ventricular end-diastolic area (LVEDA), PAWP, and CVP are also used.
In the general ICU environment, approximately 50% of hypotensive patients are fluid responsive.4 Hypovolemia is common following cardiac surgery, as a consequence of blood, urinary, and third-space fluid losses. Blood loss may be visible (seen in the chest drains) or occult (unseen in the chest, retroperitoneum, or gastrointestinal tract). Urinary losses may be substantial in the first few hours following surgery, particularly if hypothermic CPB has been employed or mannitol has been used in the circuit prime. Third-space losses are variable but may involve several liters. In one study, the average weight gain after cardiac surgery was 7.2% of body weight on the first postoperative day.5 Thus, hypovolemia can occur despite a substantial increase in total extracellular volume. In critically unwell patients, hypovolemia and systemic edema often coexist.
Diagnosis
Hypovolemia is usually obvious on the basis of clinical signs and invasive hemodynamic monitoring. However, in certain situations the clinical picture is confusing. Young patients with intact cardiovascular reflexes can have important hypovolemia without showing clinical indicators, whereas modest hypovolemia can cause profound hypotension in elderly patients who are mechanically ventilated and have blunted sympathetic tone. Hypovolemia frequently coexists with other causes of hemodynamic instability, complicating the diagnosis. The surrogates of preload, CVP and pawp, are often misleading (see Chapters 1 and Chapter 8).
Hemodynamic Monitoring.
Hypovolemia is normally characterized by hypotension in association with low atrial pressures. A patient with a CVP below 8 mmHg or a PAWP below 10 mmHg, in the presence of hypotension, is invariably fluid responsive. However, there are no absolute values for CVP and PAWP that predict fluid responsiveness,6,7 and in many circumstances patients with much higher filling pressures also benefit from fluid administration. High CVP may be caused by right ventricular dysfunction or high intrathoracic or intrapericardial pressure. A CVP above 15 mmHg is occasionally required so as to optimize cardiac output in patients with right ventricular dysfunction.4,6 Conversely, in patients with left ventricular dysfunction, CVP may be low despite circulatory overload. In cases of severe left ventricular hypertrophy, PAWP may have to be above 18 mmHg to optimize preload.4 The causes of high CVP and PAWP are listed in Tables 8-3 and 8-4, respectively.
In contrast to absolute values of CVP and PAWP, respiratory fluctuations in CVP and the arterial pressure waveform (pulsus paradoxus) are predictive of fluid responsiveness (see Chapter 8).4 A patient with a respiratory swing on the arterial waveform is invariably fluid responsive, irrespective of the atrial pressure.
Echocardiography.
However, because ventricular volume is also determined by systolic and diastolic function, there is no absolute value for LVEDA that predicts fluid responsiveness.8 In patients with normal ventricular function, euvolemia is associated with an LVEDA of 12 to 17 cm2. However, with systolic dysfunction, much higher values may be required so as to optimize stroke volume; with diastolic dysfunction, preload may be optimal at a lower end-diastolic area.
Changes in Hematocrit.
A rising hematocrit suggests that urinary losses or third-space losses are not being matched by adequate fluid resuscitation. Sudden blood loss does not lead to an immediate fall in hematocrit. However, over minutes to hours, as fluid is redistributed from the interstitium and cells, hematocrit will fall even without fluid resuscitation. Conversely, vigorous fluid administration will result in a fall in hematocrit without blood loss.
Treatment
Hypovolemia may be treated with crystalloid or colloid solutions (see Chapter 32). Neither fluid has a protective benefit against pulmonary edema or is associated with improved patient outcome.9 With crystalloid fluid, approximately one and a half times the volume of a colloid must be administered to achieve the same clinical effect.9
In critically unwell patients, the PAWP associated with optimal stroke volume may cause pulmonary edema. Therefore a balance must be struck between gas exchange and cardiac output. In mechanically ventilated patients, modest increases in positive end-expiratory pressure (PEEP) (e.g., from 5 to 10 cm H2O) may protect against pulmonary edema and allow a higher PAWP to be tolerated. Conversely, in the presence of intrapulmonary shunting, low cardiac output is associated with impaired gas exchange (see Chapter 27). Thus, in hypovolemic patients, fluid administration may improve oxygenation.
Low Systemic Vascular Resistance
Pathologic vasodilatation after cardiac surgery is most commonly a manifestation of severe inflammatory response to CPB and surgical stress (see Chapter 2). Other causes include vasodilator drug therapy, anaphylaxis, sepsis, and adrenal failure. Low systemic resistance occurs in 20% to 45% of patients after cardiac surgery,10,11 and severe vasodilatory shock occurs in 5% to 10%.11,12 Risk factors for pathologic vasodilatation include low ejection fraction, prolonged CPB, and prior treatment with ACE inhibitors.
Diagnosis
The cardinal features of systemic vasodilation are hypotension and high cardiac output. Calculated systemic vascular resistance is low (see Eq. 1-5). Atrial pressures are normal or low, but enthusiastic fluid administration may lead to high filling pressures. Diastolic blood pressure is usually low (<50 mmHg) and pulse pressure is usually high (>50 mmHg).
Warm, dilatated peripheries are not a consistent finding in vasodilatated patients in the first few hours after surgery. If the cause of the vasodilatation is systemic inflammation, there may be signs of multiorgan involvement, particularly impaired gas exchange (with evidence of pulmonary edema on chest radiograph), fever, leukocytosis, abnormal liver function, and oliguria. Serum amylase may be mildly elevated.
Treatment
Concomitant hypovolemia must be corrected, but there is little gain in boosting atrial pressures to supranormal values. Hypotension should be treated with a norepinephrine infusion titrated to an appropriate MAP. If hypotension persists despite high-dose norepinephrine (>0.3 to 0.6 μg/kg/min), vasopressin (0.01 to 0.04 units/min) may be considered. The use of methylene blue (1.5 mg/kg over 5 to 10 minutes) has been reported, and in one small series it appeared to improve survival rates in postcardiac surgery vasoplegic shock.12 As inadequate stress release of cortisol exacerbates vasoplegic shock, treatment with hydrocortisone (100 mg intravenously 8 hourly) may be considered.13 In a patient with a low ejection fraction, it is important to ensure that treatment with vasopressors does not precipitate a major fall in cardiac output.
Left Ventricular Systolic Dysfunction
Left ventricular systolic dysfunction may be acute, chronic, or a combination of both.
Chronic
Chronic left ventricular systolic dysfunction results in remodeling (see Chapter 1), usually as a consequence of myocardial infarction, chronic ischemia (hibernating myocardium), or valvular heart disease. Ejection fraction is reduced and ventricular volumes are increased. A patient with compensated heart failure may have a normal cardiac output and left atrial pressure and, despite a low ejection fraction, may have good functional capacity. Such a patient often has an uneventful postoperative course. However, decompensation may occur if: (1) there is a further myocardial insult (e.g., myocardial stunning); (2) ventricular afterload is abruptly increased (e.g., after tracheal extubation or the withholding of usual vasodilator therapy); (3) there is a requirement for increased cardiac output (e.g., a severe inflammatory response to CPB).
Acute
Acute left ventricular systolic dysfunction occurs due to myocardial ischemia, acute infarction, myocardial stunning, drugs (e.g., β blockers), or systemic inflammation. Some degree of myocardial stunning occurs in all patients after cardiac surgery.14 However, severe postoperative stunning is likely when: (1) CPB is complicated or greatly prolonged; (2) severe myocardial ischemia was present preoperatively. With acute dysfunction, ejection fraction is reduced but ventricular volumes may be relatively normal. Cardiac output is reduced, left atrial pressure is increased, and there may be signs of pulmonary congestion and tissue hypoperfusion.
Myocardial ischemia must always be considered in any patient who develops new left ventricular systolic dysfunction. The patients at the highest risk for myocardial ischemia are those undergoing coronary revascularization (see Chapter 9), but ischemia can also occur in other situations, namely, the obstruction of a coronary ostium during aortic valve replacement; the kinking or obstruction of a coronary artery following its reimplantation during aortic root replacement; damage to the circumflex coronary artery during mitral valve surgery; and damage to the septal branch of the left anterior descending coronary artery as it runs in front of the pulmonary valve during the Ross procedure (see Chapter 10).
Diagnosis
Echocardiography.
With acute dysfunction, ventricular volumes may be relatively normal. Changes in wall motion are typically regional rather than global. The left atrium may appear tense and enlarged and may show rightward bowing of the interatrial septum. With tissue Doppler, the E deceleration time is likely to be short (<140 ms) and the E:Em ratio is likely to be high (<15; see Chapter 7).
The diagnosis of postoperative myocardial ischemia rests on clinical suspicion and characteristic abnormalities on the ECG, echocardiogram, and biochemical markers (see Chapters 9 and Chapter 18).
Treatment
Preload, heart rate, and rhythm should be optimized. A heart rate of 80 to 100 beats per minute, using pacing if necessary, is appropriate for most patients. Inotropic support should then be commenced and titrated to effect. The choice of drug depends on the hemodynamic state. If cardiac output is low but blood pressure is satisfactory, an inodilator such as dobutamine or milrinone may be used. If both blood pressure and cardiac output are low, epinephrine or a combination of norepinephrine and milrinone (or dobutamine) may be used. If hemodynamic instability persists despite modest inotropic support (epinephrine 0.1 to 0.2 μ/kg/min or equivalent), insertion of an intraaortic balloon pump (IABP) should be considered (see Chapter 22). In patients with systolic ventricular dysfunction, high-dose norepinephrine can cause a precipitous fall in cardiac output and should be avoided unless cardiac output is being measured.
Combinations of inotropic drugs at high dosages (e.g., norepinephrine plus milrinone; or epinephrine/dopamine plus milrinone, and vasopressin) in conjunction with an IABP are sometimes required for severe postoperative myocardial stunning, and biventricular failure. If the hemodynamic state remains inadequate despite an IABP and combination high-dosage inotropic pharmacotherapy, and cardiac dysfunction is potentially reversible, placement of a ventricular assist device should be considered (see Chapter 22).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree