Hemodialysis Vascular Access
Anil K. Agarwal
INTRODUCTION
An ideal vascular access (VA) would provide adequate blood flow for performance of hemodialysis (HD) treatments on a consistent basis. It would be easy to create, cannulate, and maintain and should be devoid of the complications of bleeding, thrombosis, stenosis, and infection. HD was pioneered during the last century using a VA that has evolved from arterial cutdown and Scribner shunt to the Cimino fistula. Use of newer forms of VA such as arteriovenous grafts (AVGs), subcutaneous ports, and various HD catheters has become common. Despite these advances, the quest for an ideal VA is far from over.
Chronic kidney disease (CKD) of various stages afflicts over 13% of the population of the United States. In 2007, over 360,000 patients required maintenance dialysis with stage V CKD, also known as end-stage renal disease (ESRD) with over 108,000 new patients requiring dialysis, the vast majority as a result of diabetes mellitus and hypertension. Other major causes of kidney failure include various glomerulonephritides, polycystic kidney disease, and obstructive uropathy. People with failed transplant contribute a significant number to the pool of ESRD. The mortality in this population remains very high, from almost 40% during the first 3 months (particularly in older patients) to over 20% per year later. VA not only is the most important determinant of the success of HD process, it also impacts the survival on HD significantly. Specifically, there is a significant body of data showing that arteriovenous fistula (AVF) is associated with the best outcomes with catheter access being associated with a high degree of morbidity and mortality. Considering these factors, the Kidney Disease Outcomes Quality Initiative (K/DOQI) recommends placement of AVF as a priority in patients who may need permanent dialysis. In the United States, Fistula First Initiative, using its “Change Concepts,” has been successful in increasing the prevalence of AVF among the prevalent ESRD patients from approximately 30% in 2003 to over 50% in 2009, although the goal of 66% prevalent AVF by 2010 is yet to be achieved.
As the number of patients requiring HD has increased, the number of procedures for VA has increased as well. Access-related procedures are the most common vascular surgery procedure done in the United States. Many of these are done for initiation of dialysis; however, since the lifespan of an access site is limited, many are done to revise existing sites. VA-related complications, especially sepsis and thrombosis, are responsible for 15 to 20% of hospitalizations in the ESRD patients undergoing HD. Access-related procedures cost over $1.8 billion annually in economic costs.
This chapter provides an overview of several important issues related to VA, including making a choice of VA, planning, creation, and maintenance of VA.
Indications for Dialysis and Vascular Access Creation
Dialysis for acute renal failure or CKD is needed to treat the uremic syndrome, medically refractory volume overload, hyperkalemia, and acidosis. Drug overdose and intoxications can sometimes need dialysis.
CHOICE OF VASCULAR ACCESS
The choice of VA for HD depends on several clinical factors. These include the timing (when dialysis will be started), duration (how long it will be continued), and the suitability of the patient’s vasculature. The ideal site should have easy access to the circulation, durability, and limited complications and should provide effective dialysis. The access that comes closest to reaching this goal is the autogenous AVF. Despite these obvious advantages, until recently, the most common type of access used in practice has been the polytetrafluoroethylene (PTFE) graft. Although this prosthetic graft suffers from high rates of thrombosis and failure, it was used more commonly than AVF owing to such factors as delayed referral for AVF placement, unsuitable veins, and greater technical expertise required for AVF placement. Additionally, there is a significant risk of failure of maturation of AVF. However, once mature, AVF requires minimal intervention and is associated with the lowest incidence of infection and expense. Catheters and ports constitute the other major type of access but are complicated by a high incidence of infection and thrombosis.
Timing of access placement is immensely important as the maturation time for an AVF is 4 to 6 weeks. The waiting time for cannulation of AVF after placement also varies widely. Given the high rates of delayed or failed maturation, an AVF should be created 6 to 12 months before anticipated dialysis. This requires early referral to nephrologists and surgeons. Once an AVF is planned, it is important to protect the veins of the nondominant arm. The nondominant arm is preferred so that the dominant arm is free during dialysis. Ultimately, however, the quality of the vessels takes priority. AVGs are usually ready for use within 2 to 4 weeks (which accounts partly for their wider usage), are less likely to fail early, but require higher numbers of intervention and have less longevity than AVF. Catheters can be used instantly but are complicated by thrombosis, infection, and central venous stenosis.
ANATOMIC CONSIDERATIONS
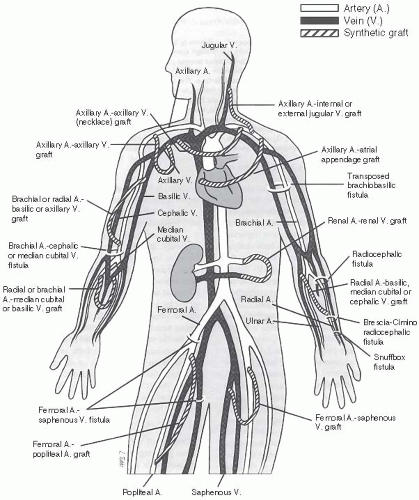
Figure 27.1 shows various anatomic sites and most plausible configurations for AVFs and synthetic grafts. AVFs can be in side-to-side (vein to artery), end-to-side, or end-to-end pattern. The basilic and cephalic veins are the most important veins used for VA, because they are subcutaneous and more accessible than deep veins. It is also possible to use perforating
veins or transpose veins in the upper extremity. Clinical variables that may predict failure of an AVF include advanced age, female gender, diabetes, and obesity, often due to small vessel size. However, presence of these factors does not necessarily preclude AVF placement and maturation.
veins or transpose veins in the upper extremity. Clinical variables that may predict failure of an AVF include advanced age, female gender, diabetes, and obesity, often due to small vessel size. However, presence of these factors does not necessarily preclude AVF placement and maturation.
VASCULAR ACCESS PLANNING
Physical Examination
A careful preoperative physical examination is extremely important in planning access placement. The inflow artery and the outflow vein must
be examined. The inflow artery should be able to provide adequate flow without rendering the extremity ischemic. Physical exam should involve palpation of the brachial, radial, and ulnar pulses. Blood pressures in the arms should be within 20 mm Hg of each other. The Allen test should also be performed in all patients. The examination of the venous system involves inflation of a blood pressure cuff to 5 mm Hg above diastolic pressure for less than 5 minutes. The cephalic vein should be relatively straight from the wrist to the antecubital space. Accessory veins should be identified so that they can be ligated during surgery as these can delay maturation of the fistula by siphoning blood from the outflow vein.
be examined. The inflow artery should be able to provide adequate flow without rendering the extremity ischemic. Physical exam should involve palpation of the brachial, radial, and ulnar pulses. Blood pressures in the arms should be within 20 mm Hg of each other. The Allen test should also be performed in all patients. The examination of the venous system involves inflation of a blood pressure cuff to 5 mm Hg above diastolic pressure for less than 5 minutes. The cephalic vein should be relatively straight from the wrist to the antecubital space. Accessory veins should be identified so that they can be ligated during surgery as these can delay maturation of the fistula by siphoning blood from the outflow vein.
Vessel Mapping
Emphasis should be placed on preoperative vessel mapping, especially in high-risk cases. Duplex studies are commonly used to provide the surgeon a roadmap for creation of a successful AVF. Both the inflow arteries and the veins should be examined. Factors that are considered favorable include minimum venous and arterial lumen diameters of at least 2.5 mm and 2.0 mm, respectively; absence of stenoses; continuity of the vein with the deep venous system; and absence of calcification of the inflow artery. Preoperative venography may be appropriate in those with a history of previous central venous cannulation to demonstrate patency of central veins, which is difficult to detect with duplex study. Use of a small amount of low osmolar radiocontrast agents has been shown to be safe without the occurrence of acute renal failure even in the presence of advanced renal insufficiency. Use of vessel mapping has been shown to be associated with a higher rate of AVF placement, although this does not translate into a higher maturity rate of AVF.
CREATION OF ARTERIOVENOUS FISTULA
The first AVF should be placed as distally in the forearm as possible to maximize the ability to secure future AVFs. This consideration has to be tempered by the realization that the distal vessels are smaller than proximal vessels and less likely to be of adequate caliber. The radio cephalic fistula is the preferred first fistula. It has been modified from its original configuration, which was a side-to-side anastomosis, as this configuration led to excessive venous congestion in the hand from venous hypertension. To avoid this, the vein is ligated distally and an end-to-side anastomosis is created. The second choice is a proximal forearm fistula. A brachiobasilic fistula is technically more time-consuming and has a higher rate of thrombosis than the standard brachiocephalic fistula. Its advantage is that it has a lower early failure rate. Another possible option would be a prosthetic graft as this will allow time for the proximal veins to dilate, so that when the prosthetic graft fails, the proximal veins will be available and suitable for an AVF. The risk of such a strategy is that the proximal veins may become stenotic and unsuitable for AVFs.
Secondary AVFs (SAVFs) are defined as the fistulae created after the failure of previous VA in the same or another extremity. SAVFs provide an important, though underutilized, strategy to improve prevalent AVF rates and must be considered in those with a failing existing VA.
FAILURE OF MATURATION OF ARTERIOVENOUS FISTULA
Soon after the creation of AVF, there is a marked increase in blood flow via the newly created vascular circuit. This is attributed to venous remodeling with dilatation and “arterialization” as well as to arterial dilatation under the influence of changes in shear stress and cytokine expression profile. There are a number of reasons for the AVF’s failure to mature. These commonly include presence of accessory veins or stenosis of venous outflow. Presence of stenosis of the juxta-anastomotic segment (the so-called “swing segment”) of the outflow vein is not an uncommon finding. Additionally, there may be failure of arterial dilatation. The exact mechanism for nonmaturation of AVF in many of the seemingly “adequate” vessels remains unclear at this time.
After the AVF is created, it is important to monitor both the extremity and the fistula. The presence of accessory veins is sought by occluding the fistula with a finger. The presence of a stenosis is suggested by a very strong pulse upstream followed by a decrease in intensity or disappearance of the pulse beyond the stenosis. Also, a normal fistula has a continuous thrill with a soft compressible pulse. The direction of blood flow is determined by occluding the fistula and feeling for a pulse on either side of the occlusion. The inflow side is the one with the pulse.
In general, if an AVF does not show signs of maturation within a month, investigation must be undertaken to identify a cause. Ultrasound or venography should be used to identify accessory veins, stenosis of outflow vein, stenosis at the anastomosis, and CVS. It is important to recognize that many AVFs with failure to mature can be salvaged using percutaneous intervention, including angioplasty of stenosis or coiling of accessory veins.
PROSTHETIC ARTERIOVENOUS GRAFT PLACEMENT CONSIDERATIONS
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree