Hemodialysis Shunts
Dierk Vorwerk
Percutaneous procedures for hemodialysis shunts are becoming increasingly important for interventional radiologists. A growing number of patients with renal insufficiency are enrolled in dialysis programs, the majority of them undergoing hemodialysis. In the West, this affects some 150 to 200 persons per million inhabitants.
Given the increase in patients’ life expectancy, maintaining access to the vascular system continues to be a problem.1 For example, the number of long-term functioning shunts is estimated to be 15%.1 Primary patency of hemodialysis shunts is low: Approximately 65% of Brescia-Cimino shunts and 50% of polytetrafluoroethylene (PTFE)-covered shunts exhibit primary patency after 1 year, and the numbers sink after 2 and 4 years to 60% and 45% for the Brescia-Cimino shunts and to 43% and 10% for the PTFE-covered shunts.2
In Europe, arteriovenous Brescia-Cimino shunts are the preferred primary shunts, used in conjunction with the radial artery and veins of the lower arm. Autologous veins in the proximal lower arm and elbow region are generally preferred, even for renewed shunt application. PTFE-covered shunts, utilizing foreign-body implants such as Gore-Tex segments, are mainly used where the vascular conditions are very complicated and unfavorable. In the United States, in contrast, PTFE-covered shunts are used much more frequently as primary shunts, often accounting for the majority of shunts in a given patient population.
The choice of access site, the indications, and the interventional technique employed depend on the nature and location of the shunt, the site of the lesion, and the nature of the obstruction. As a rule, the presence of a stenosis or a thrombus, the type of shunt, and the location of the obstruction determine the type of technique used.
The objective of this chapter is to describe the technical options for percutaneous interventions for different types of shunt problems. The surgical alternatives are not discussed. It is important to mention, however, that comparative studies have not produced any indication of significant differences between surgical and percutaneous procedures with regard to immediate or long-term outcome.
Diagnosis of Shunt Problems
Sufficient diagnosis to classify the location and nature of the disturbance is necessary before performing any intervention on hemodialysis shunts. Simple palpation of the brachial and radial arteries and the shunt veins with and without stasis provides important information about the condition of the veins and the presence of stenotic or thrombotic segments.3
High-quality, noninvasive, morphologic diagnosis and, to a lesser extent, functional diagnosis of hemodialysis shunts can be achieved with various sonographic methods. Real-time sonography and color-coded duplex sonography are superior to angiography in detecting thromboses and should be employed for shunt thromboses in order to image the extent of the clot.4
Angiographic study is, however, essential in the actual planning of an intervention. It always consists of complete representation of the supplying arteries (as far as possible), and of the draining veins up to the superior vena cava. The purpose of complete representation is to rule out the existence of multiple lesions.
Transbrachial Fine Needle Angiography
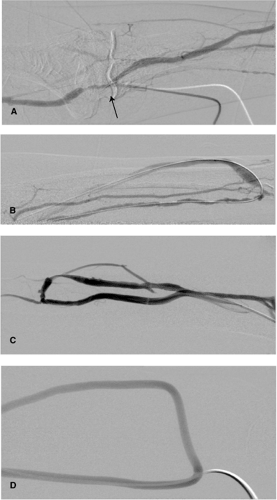
Transbrachial angiography (Fig. 13-1 A, B) provides the best access to all shunt connections from the brachial artery distal to the elbow or any of its branches. It can be employed for almost all
Brescia-Cimino shunts and some PTFE-implant shunts. Under local anesthesia, a retrograde puncture of the palpable brachial artery is performed using a fine 22-gauge puncture needle with a plastic cover according to the Seldinger technique at elbow level where the artery passes under the aponeurosis of the biceps muscle. The plastic cover is best inserted into the artery as far as the Luer connector in order to reach a safe position. This occasionally makes it necessary to use an 0.018-in. guidewire. The synthetic needle is then fixed with sterile adhesive tape, and a thin extension tube with lock fitting (e.g., Perfusor tubing, Braun, Melsungen, Germany) is attached for contrast medium administration. Subsequently, a diluted solution of contrast medium is manually injected to permit visualization of the entire course of the shunt and long sections of the brachial artery using retrograde, digital subtraction angiography (one to two images per second). As a rule, 20 to 40 mL contrast medium (saline: contrast 1:1) is sufficient.
Brescia-Cimino shunts and some PTFE-implant shunts. Under local anesthesia, a retrograde puncture of the palpable brachial artery is performed using a fine 22-gauge puncture needle with a plastic cover according to the Seldinger technique at elbow level where the artery passes under the aponeurosis of the biceps muscle. The plastic cover is best inserted into the artery as far as the Luer connector in order to reach a safe position. This occasionally makes it necessary to use an 0.018-in. guidewire. The synthetic needle is then fixed with sterile adhesive tape, and a thin extension tube with lock fitting (e.g., Perfusor tubing, Braun, Melsungen, Germany) is attached for contrast medium administration. Subsequently, a diluted solution of contrast medium is manually injected to permit visualization of the entire course of the shunt and long sections of the brachial artery using retrograde, digital subtraction angiography (one to two images per second). As a rule, 20 to 40 mL contrast medium (saline: contrast 1:1) is sufficient.
This procedure is simple, is infrequently associated with complications, and can be performed on an outpatient basis. Access to the artery can be maintained if a subsequent intervention is planned, in order to monitor the success of the intervention. Care should be taken that a puncture performed prior to the intervention does not puncture the artery in the immediate vicinity of the basilic vein, which is often adjacent, to avoid damage to the potential venous access.
Transfemoral Angiography
Transfemoral selective brachial angiography via the subclavian artery is performed only in exceptional cases, including cases with high proximal arterial stenoses which cannot be adequately assessed by the retrograde brachial approach or duplex sonography or cases considered for angioplasty. As a rule, the transfemoral approach should not be performed on an outpatient basis.
Shunt Imaging (Shuntogram)
Direct puncture of the venous portion of the shunt for diagnostic purposes can be performed in PTFE-covered shunts with anastomosis to the brachial artery or above the elbow joint and for any type of shunt in the upper arm (Fig. 13-1 C,D). Using the fine-needle approach, the PTFE-implant loop or the vein is best punctured in the direction of flow, since the access can be also used for intervention. The arterial shunt portion can then be imaged using retrograde overflow angiography after compression of the venous drainage. Puncture should not be made too far from the arterial anastomosis to allow high quality imaging of the arterial shunt arm and to avoid complications.
In cases considered for a subsequent intervention, however, the puncture site should not be too close to the site of the intervention to allow secure sheath placement and endovascular instrumentation.
Diagnostic angiography should not be performed in patients with thrombosis of a PTFE-covered shunt since there is always complete closure of the shunt ranging from the arterial to the venous anastomosis.
Imaging of native upper arm shunts is best done in an antegrade direction in the section of the vein near the anastomosis where the pulse is still palpable, such that this access can be enlarged and used for the subsequent intervention, if needed. In these cases, any puncture of the brachial artery should be avoided altogether.
Other Imaging Techniques
Although other imaging modalities such as MR and CT angiography also allow shunt monitoring, they are too cumbersome and too expensive to become widely used for shunt diagnostics in real-life clinical practice. In cases with difficult puncture, sonography may be helpful.
Access
The location and the type of shunt determine which access is appropriate.
Native Lower Arm Shunts
As a rule, venous access is chosen for Brescia-Cimino shunts. The shunt vein is punctured with the patient wearing a compression bandage. A large-bore cannula and a regular 0.035-in. guidewire should be used in large veins. In small and poorly palpable veins, the micropuncture is recommended using a 22-gauge needle and a 0.018-in. guidewire. Following the successful puncture, a 16-gauge plastic cannula can be easily inserted over the guidewire into the shunt vein and advanced while being carefully rotated. Imaging must confirm that the thin wire has not been kinked to prevent breaking. Once correct intraluminal position of the plastic cannula has been confirmed, the stylet and guidewire should be replaced by a 0.035-in. guidewire, preferably hydrophilically coated. Retrograde venous puncture is performed in lesions near anastomoses (i.e., distal venous lesions), stenotic anastomoses, and distal arterial stenoses.
Transbrachial arterial access may be necessary in exceptional cases if the operator does not succeed in crossing a stenosis near the anastomosis from the venous access. In these cases, the brachial artery is punctured in an antegrade fashion at the height of the elbow joint and, after careful probing of the artery supplying the shunt — normally the radial artery — a 4F catheter is inserted. The stenosis is probed using the guidewire reinforced by a dilatation catheter. Once the stenosis has been crossed the guidewire tip is caught by a snare introduced from the vein and guided out via the venous access. To minimize the risk of arterial injury the intervention is performed from the venous access.
For stenoses in the proximal segments of the draining veins, the shunt vein is punctured in an antegrade fashion at a more peripheral puncture site. If such a proximal stenosis cannot be probed and crossed from the peripheral site or probing is prevented by complicating dissections or perforations, then the crossing should be tried by approaching the target lesion from a second venous access established by a retrograde puncture of the target vein.
Native Upper Arm Shunts
In lesions close to the anastomosis, the brachiocephalic vein is punctured in a retrograde fashion at a far proximal site, i.e., downstream from the target lesion. If the lesion is located close to the trunk (more proximally), the brachiocephalic vein is punctured close to the anastomosis. A double access is often advisable in thromboses of upper arm shunts, whereby a retrograde puncture can be made first in the thrombosed vein to perform partial thrombectomy on one segment to be complemented by a thrombectomy of the rest of the thrombus from a second antegrade access.
Ipsilateral puncture of the internal jugular vein with retrograde probing of the target lesion has been considered an alternative access to the brachiocephalic vein.
Implanted Shunts
In PTFE-implant shunts, the puncture site in the loop depends on whether the stenosis is located in the arterial (retrograde) or venous portion of the anastomosis or in the draining veins of the upper arm. A diagnostic puncture made according to the micropuncture technique described earlier can usually be enlarged and used for the intervention.
In loop shunts, the apex of the shunt arch is an advantageous puncture site. After the dilatator has been inserted into the catheter, the guidewire can be advanced and directed into either the arterial or the venous shunt portion, making it possible to access both arms of the shunt from one site.
In straight grafts, access should be via the implant itself and is best performed in the direction of flow. Two access sites are frequently necessary in all types of shunts, particularly if thrombectomy has been intended, particularly if the thrombus extends close to the arterial site of the graft anastomosis.
Central Veins
In lesions of the proximal cephalic and subclavian veins, preferred access is from the arm using the shunt vein. In rare cases, when large balloons or stent devices are used, transfemoral venous access is selected to avoid injury of the shunt veins. Note, however, that a 10F catheter can be inserted into an arm vein safely. Combined cannulation of the femoral and brachial veins is occasionally necessary to achieve continuous monitoring of the stent implants in central veins. In critical locations, stents can be placed over a guidewire forming a loop through the brachial and femoral vein in order to provide support for the transvenous segment and possibly prevent stent embolization into the pulmonary circulation.
Arterial Stenoses
For proximal lesions of the arterial arm of the shunt, access can be achieved either via retrograde brachial artery puncture or—in the case of the upper arm synthetic shunts—by a retrograde transvenous puncture. If the brachial pulse is weak, the micropuncture technique is occasionally appropriate after the stenosis has been located using a pocket Doppler. Transfemoral arterial access for dilatation is only indicated in the unusual case of arterial stenoses of the proximal brachial artery.
Shunt Stenoses
Since shunt stenoses are frequently the cause of subsequent shunt thromboses, they should be diagnosed and removed promptly. They present as increased venous counterpressure during hemodialysis, increased recirculation, or lengthened hemostasis, or, if the stenosis is located near the anastomosis, as venous collapse due to suction or insufficient arterial flow.
Although shunt stenoses can develop in any venous segment, with Brescia-Cimino shunts they are frequently located at or close to the arteriovenous anastomosis and the puncture segment, while with PTFE-covered shunts they are more frequently located proximal to the venous anastomosis.
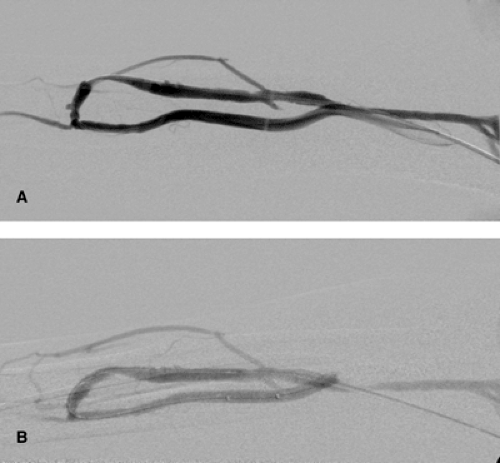
The procedure of choice is balloon dilatation (Fig. 13-2). A 5F low-profile catheter should be selected allowing a smooth navigation and tracking of curves. Alternatively, in the afferent
radial artery, a combination of a catheter with a 0.035-in. hydrophilic guidewire can be used. A 6F sheath is usually sufficient.
radial artery, a combination of a catheter with a 0.035-in. hydrophilic guidewire can be used. A 6F sheath is usually sufficient.
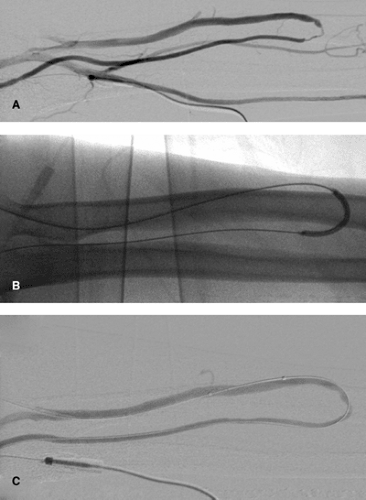
Using the retrograde approach to the target lesion prior to introducing the balloon catheter, a catheter and hydrophilic guidewire should pass the lesion first. The guidewire should be placed deep into the artery via the arteriovenous anastomosis. Using this technique, it is possible to dilate distal arterial lesions (Fig. 13-3), stenoses of the anastomosis, and distal venous stenoses. In my own experience, the use of conventional balloon systems is usually sufficient. Special balloon systems with short shafts do not offer any particular advantages.
It is important to note that extremely resistant stenoses are encountered relatively frequently in shunt veins and that they can resist balloon pressures as high as 15 bar and higher. In such cases, high-pressure balloons with an average burst pressure of 20 bar or cutting balloons (BSIC, Boston, MA) are employed.
Other special types of resilient stenoses that can limit the outcome of balloon dilatation. They include collapsing stenoses, which collapse upon themselves immediately after dilatation. Furthermore, increased flow volume can lead to venous dilatation and elongation, which can result in kink stenoses, especially in the proximity of stenotic venous valves. Although these stenoses can be well dilated, they recoil immediately after balloon deflation. They are hardly amenable to a plain balloon percutaneous transluminal angioplasty (PTA); stents may be helpful in the upper arm shunts. Dilatations of central veins may require large balloons, 12 mm to 16 mm in diameter.
During the intervention, 2500 to 5000 U heparin should be administered intravenously. Careful provision should be taken for hemodialysis following the intervention no later than the next day. During hemodialysis, full heparinization is provided but no anticoagulatory treatment is required subsequently.
Indications for Intervention in Stenoses
Percutaneous intervention of stenotic hemodialysis shunts is always indicated when a stenosis is more than 40% to 50% of the diameter. Sullivan and coworkers reported significant reduction in blood pressure with stenoses >40% diameter,5,6 which indicates the hemodynamic significance of even moderately sized stenoses. In hemodynamic terms, it is therefore grade shunt stenoses can become significant in the presence of a low peripheral resistance.
Type, length, and location of a stenosis do not predict whether the stenosis will respond to balloon dilatation. Exceptions to this rule are central venous stenoses and long arterial lesions of the supplying lower arm artery, which generally do not respond favorably to balloon dilatation alone.
Whereas stenting is indicated for central stenoses, stenting may not be an alternative in long arterial stenoses. If surgical shortening of the shunt and reimplantation are not possible, then a balloon dilatation must be attempted, regardless of unfavorable prognosis. Interestingly, Beathard et al. were not able to confirm the frequently discussed limitations of PTA in long lesions (>6 cm) or stenoses inside PTFE-covered shunts.7
Thus, in most cases, stenoses resistant to dilatation can only be identified during balloon inflations.
Results of Balloon Dilatation
The results of plain balloon angioplasty are in most cases technically satisfactory. In large series, the reported success rate ranged between 82% and 94%, and the complication rates ranged from 2% to 6%.7,8,9,10 The long-term results, in contrast, are less convincing. Gmelin and Karnel10 reported a markedly reduced patency over time, dropping from 75% at 6 months to 34% at 2 years. Glanz et al.9




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree