(1)
Project-team INRIA-UPMC-CNRS REO Laboratoire Jacques-Louis Lions, CNRS UMR 7598, Université Pierre et Marie Curie, Place Jussieu 4, 75252 Paris Cedex 05, France
Abstract
Hematopoietic cells, or hemopoietic cells, represent bone marrow-derived cell types that circulate in blood (including mature cell types and their precursors). Hematopoietic cells are categorized into myeloid cells (basophils, eosinophils, neutrophils, erythrocytes, thrombocytes, monocytes and macrophages, and mastocytes) and lymphoid cells (Blymphocytes, various types of Tlymphocytes, which are the only hematopoietic cells that can be generated elsewhere than in the bone marrow, and natural killer [NK] cells, that are cytotoxic lymphocytes also called large granular lymphocytes).
Hematopoietic cells, or hemopoietic cells, represent bone marrow-derived cell types that circulate in blood (including mature cell types and their precursors). Hematopoietic cells are categorized into myeloid cells (basophils, eosinophils, neutrophils, erythrocytes, thrombocytes, monocytes and macrophages, and mastocytes) and lymphoid cells (B lymphocytes, various types of T lymphocytes, which are the only hematopoietic cells that can be generated elsewhere than in the bone marrow, and natural killer [NK] cells, that are cytotoxic lymphocytes also called large granular lymphocytes).
Stromal cells is another collective term for different cell types in a given tissue at a given time that do not pertain to functional components of this tissue, i.e., parenchymal cells. In particular, the bone marrow stroma yields a hematopoietic microenvironment that consists of various cell types, such as mesenchymal progenitors, fibroblasts, adipocytes, monocytes and macrophages, endothelial and smooth muscle cells, T-lymphocytes, among others. These cells can support short- and long-term hematopoiesis via membrane-bound and secreted hematopoietic factors that operate on hematopoietic stem and progenitor cells.
Hematopoietic stem cells produce by mitosis either stem cells of the same type (self-renewal) or progenitors leading to precursor, immature, and mature blood cells. In steady state, most stem cells are dormant, whereas few are active [37]. Hematopoietic stem cell self-renewal is regulated by intrinsic and extrinsic signals.
Blood-cell progenitors and mature cells of the granulocytic, monocytic, megakaryocytic, and erythroid lineages can be generated from differentiated cells. For example, human dermal fibroblasts that bypass the pluripotent stem cell state can give rise to multipotent hematopoietic progenitors. These fibroblasts synthesize predominantlyoctamer-binding transcription factor Oct4, or POU domain, class-5, homeodomain-containing transcription factor POU5F1, as well asPTPRc phosphatase, or panhematopoietic marker CD45, during the multipotent reprogramming [38].
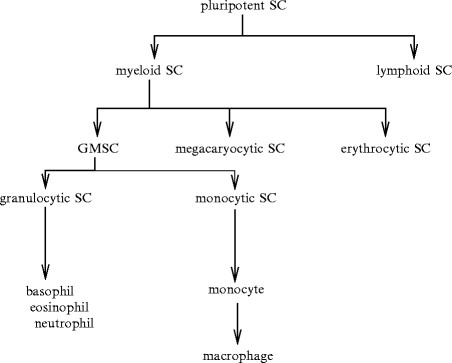
The turnover of hematopoietic cells1 is estimated to be about 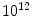 cells per day [39]. All blood cell types are produced in the bone marrow frompluripotent stem cells (PSC; Fig. 2.1). Hematopoietic stem cells and derived progenitor cells yield blood resupply during the entire life. Pluripotent stem cells give birth via multi- and unipotent stem cells (USC) to the different lineages via proerythroblasts and erythroblasts, megakaryoblasts, myeloblasts and promyelocytes, and lymphoblasts.
cells per day [39]. All blood cell types are produced in the bone marrow frompluripotent stem cells (PSC; Fig. 2.1). Hematopoietic stem cells and derived progenitor cells yield blood resupply during the entire life. Pluripotent stem cells give birth via multi- and unipotent stem cells (USC) to the different lineages via proerythroblasts and erythroblasts, megakaryoblasts, myeloblasts and promyelocytes, and lymphoblasts.
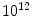 cells per day [39]. All blood cell types are produced in the bone marrow frompluripotent stem cells (PSC; Fig. 2.1). Hematopoietic stem cells and derived progenitor cells yield blood resupply during the entire life. Pluripotent stem cells give birth via multi- and unipotent stem cells (USC) to the different lineages via proerythroblasts and erythroblasts, megakaryoblasts, myeloblasts and promyelocytes, and lymphoblasts.
cells per day [39]. All blood cell types are produced in the bone marrow frompluripotent stem cells (PSC; Fig. 2.1). Hematopoietic stem cells and derived progenitor cells yield blood resupply during the entire life. Pluripotent stem cells give birth via multi- and unipotent stem cells (USC) to the different lineages via proerythroblasts and erythroblasts, megakaryoblasts, myeloblasts and promyelocytes, and lymphoblasts.
Fig. 2.1
Hematopoiesis with a focus on granulocytic and monocytic lineages (SC: stem cell, GMSC: granulocyte–monophage stem cell). After birth, hematopoiesis occurs in the bone marrow. Under the influence of stimulators, multipotent stem cells of the bone marrow differentiate into progenitors (committed stem cells). The first differentiation leads to myeloid and lymphoid stem cells. The progenitors undergo several divisions to give rise to stem cells with limited differentiation potential, the specific progenitors and precursors of a single blood cell lineage. Lymphoid stem cells (progenitors) produce B and T lymphocytes. Myeloid stem cells, or colony-forming unit CFUgemm (granulocyte–erythroid–macrophage–megakaryocyte) give birth to progenitors of many lineages (granulocytic, monocytic, erythrocytic, and megakaryocytic). Generator CFUgemm leads to more differentiated progenitors: (1) CFUgm (granulocyte–macrophage) that give rise to CFUg (granulocyte) and CFUm (macrophage); (2) CFUeo (eosinophil); (3) CFUb (basophil); (4) CFUe (erythroid); and (5) CFUmeg (megakaryocyte). Precursors have lost self-renewal capacity. They include myeloblasts, monoblasts, lymphoblasts, proerythroblasts, and megakaryoblasts. Several shared modifications characterize cell maturation: reduction in cell size and nucleocytoplasmic ratio and chromatin condensation. Precursors lead to mature blood cells after 3 to 5 divisions according to the cell lineage (one precursor can give birth to up to 32 daughter cells). Mature blood cells enter blood. Lymphocytes and monocytes can differentiate in tissues after their blood journey. Hematopoiesis is composed of several compartments: (1) multipotent stem cells such as colony-forming unit-spleen (CFUs), (2) progenitors, (3) precursors, and (4) mature cells (granulocytes, monoocytes, lymphocytes, erythrocytes, and thrombocytes).
2.1 Hematopoietic Stem Cells
Hematopoietic stem cells (HSC) generate a set of progenitors for erythroid, lymphoid, megakaryocytic, and myeloid lineages. Another type of multipotent cells resides in the bone marrow — mesenchymal stem cells — that can differentiate into osteoblasts, chondrocytes, and adipocytes. Most primitive hematopoietic stem cells are quiescent.
Two subpopulations of hematopoietic stem cells exist: (1) long-term quiescent (reserve) and (2) active hematopoietic stem cells [40]. In the bone marrow, endosteal regions composed of osteoblasts, osteoclasts, as well as vascular and CXCL12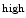 reticular cells, and central regions that lack osteoblasts form inhibitory and stimulatory (proliferative) zones, respectively. These 2 adjoining, but separate regions enable the coexistence of 2 different subpopulations (states) of hematopoietic stem cells.
reticular cells, and central regions that lack osteoblasts form inhibitory and stimulatory (proliferative) zones, respectively. These 2 adjoining, but separate regions enable the coexistence of 2 different subpopulations (states) of hematopoietic stem cells.
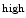 reticular cells, and central regions that lack osteoblasts form inhibitory and stimulatory (proliferative) zones, respectively. These 2 adjoining, but separate regions enable the coexistence of 2 different subpopulations (states) of hematopoietic stem cells.
reticular cells, and central regions that lack osteoblasts form inhibitory and stimulatory (proliferative) zones, respectively. These 2 adjoining, but separate regions enable the coexistence of 2 different subpopulations (states) of hematopoietic stem cells.The pool of hematopoietic stem cells comprises clonal subtypes with different lineage potential and self-renewal capacity.Aging affects the function and composition of mature blood cell compartments with a decline in lymphoid lineage potential and increased myeloid lineage commitment [41]. The gene program that specifies lymphoid fate is downregulated with age. Myeloid-biased hematopoietic stem cells produce high levels of signaling lymphocytic activation molecule family memberSLAMF1 (SLAMF1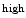 HSCs), or CD150 (Table 2.1), possess a stronger self-renewal potential, and predominate the stem cell pool in the aging body, whereas those that generate lower levels of SLAMF1 (SLAMF1
HSCs), or CD150 (Table 2.1), possess a stronger self-renewal potential, and predominate the stem cell pool in the aging body, whereas those that generate lower levels of SLAMF1 (SLAMF1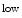 HSCs) have a balanced lineage output.
HSCs) have a balanced lineage output.
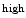 HSCs), or CD150 (Table 2.1), possess a stronger self-renewal potential, and predominate the stem cell pool in the aging body, whereas those that generate lower levels of SLAMF1 (SLAMF1
HSCs), or CD150 (Table 2.1), possess a stronger self-renewal potential, and predominate the stem cell pool in the aging body, whereas those that generate lower levels of SLAMF1 (SLAMF1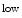 HSCs) have a balanced lineage output.
HSCs) have a balanced lineage output.Table 2.1
Members of the signaling lymphocytic activation molecule family (SLAMF) of immune receptors, or cluster of differentiation CD2 receptor family. They are synthesized in various subsets of immunocytes (T, B, and NK cells; BLAME: B-lymphocyte activator macrophage-expressed membrane protein; BLAST: B-lymphocyte activation surface marker; CD2F: CD2 family member; CD84H: CD84 homolog; CRACC: CD2-like receptor activating cytotoxic cells; CS: CD2 subset; Ly: lymphocyte surface antigen; NAIL: NK-cell activation-inducing ligand; NKR: NK-cell type-1 receptor; nLy: novel lymphocyte antigen; NTBA: natural killer-, T-, and B-cell antigen; SF: signaling lymphocytic activation molecule family member).
Member | Other aliases |
|---|---|
SLAMF1 | CD150, SLAM |
SLAMF2 | CD48, BLAST1 |
SLAMF3 | CD229, Ly9 |
SLAMF4 | CD244, NAIL, NKR2B4 |
SLAMF5 | CD84, Ly9β |
SLAMF6 | CD352, Ly108, NTBA, SF2000 |
SLAMF7 | CD319, nLy9, CRACC, CS1 |
SLAMF8 | CD353, BLAME |
SLAMF9 | CD2F10, CD84H1, SF2001 |
Hematopoiesis depends on hematopoietic stem cell survival, self-renewal, and differentiation, hence mainly on the balance between quiescence (reserve) and cell division (regeneration of its pool and blood tissue). Lifetime hematopoiesis indeed relies on the capacity of hematopoietic stem cells to self-renew and to generate blood cells according to the body’s needs. Blood homeostasis, hence hematopoiesis, requires long-term retention of hematopoietic stem cells in quiescence to maintain hematopoietic regenerative capacity. In the absence of a need of strong blood regeneration, the bone marrow, which is the site of blood cell formation from hematopoietic stem cells, maintains the circulating cell pool because mature blood cells continuously undergo senescence with a given degradation rate.
Tissue regeneration depends, at least partly, on stem cells, hence on their metabolic regulation. Hematopoietic stem cells are highly sensitive to energetic and oxidative stress; they shift between quiescence and proliferation according to the context.Kinase STK11, or LKB1, and its substrateAMPK coordinate metabolism with cell fate, thereby adapting cellular energetics with stem cell maintenance or tissue regeneration. The primary function of LKB1 in adult tissues is its inhibition of cell division, hence preventing tissue overgrowth. Enzyme LKB1 also controls cell survival and proliferation, as well as mitochondrial function and energy homeostasis in hematopoietic stem cells [42]. Inactivation of and deficiency in LKB1 in adult mice causes loss of HSC quiescence and subsequent depletion of all hematopoietic derived cells, as well as mitochondrial defects, alterations in lipid and nucleotide metabolism, and depletion of cellular ATP [42–44]. Hematopoietic stem cells relies more strongly on LKB1 for cell cycle regulation and survival than committed hematopoietic progenitor and precursor cells [42, 44]. However, LKB1 is needed for HSC maintenance via AMPK-dependent and -independent mechanisms [42, 43]. Metabolic sensor LKB1 indeed controls chromosome stability in HSCs via an AMPK-independent process. The metabolic control in hematopoietic stem cells yields an additional metabolic checkpoint of the cell division cycle. The bone marrow niche controls by sending proper cues the activity of hematopoietic stem cells during regenerative hematopoiesis.
The transcriptional regulation of cell quiescence relies on cell cycle regulators (e.g., P53, retinoblastoma protein, cyclin-D, andcyclin-dependent kinase inhibitors CKI1a and CKI1b) and factors with specific functions in hematopoietic stem cells (e.g.,early growth response factor EGR1,forkhead box protein FoxO,DNA sequence GATA-binding protein GATA2, growth factor-independent transcription repressor GFI1, andRunx1).2 The transcription factor, nuclear receptor-related protein NuRR1, or NR4a2, prevents the entry into the cell cycle [46]. Factor NR4a2 may upregulate cyclin-dependent kinase inhibitor CKI2c.
Connexin-43, a constituent of gap junctions, is synthesized in hematopoietic stem and progenitor cells. However, it is downregulated during the differentiation of hematopoietic stem cells to progenitors. Connexin-43 regulates the content ofreactive oxygen species inside stressed hematopoietic stem cells, as it enables ROS transfer to supporting stromal cells in the hematopoietic environment [45]. Whereas low ROS levels are needed for cell activity (Vol. 4 – Chap. 10. Other Major Signaling Mediators), a sustained increase in ROS production during stress (and aging) can prevent self-renewal and cause senescence. Hyporegenerative capacity and cell cycle arrest can result from high levels of ROS and activation of P38MAPK and FoxO1 agents.
The number of hematopoietic stem cells is assessed to be of the order of 10000 cells. However, the blood cell production rate varies with mammal size. In addition, the larger the species size, the faster the proliferation rate. The size of the hematopoietic stem cell pool can be associated with allometric scaling, which is defined by a simple power law between a given observable y and the mass m of the organism:3
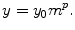
The number of active stem cells in adult mammals evaluated by measuring the number of circulating reticulocytes scales with body mass with exponent 3/4 [47].
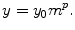
(2.1)
2.1.1 LSK Hematopoietic Stem Cells
Hematopoietic stem cells belong to a bone marrow cell population that expresses high levels of stem cell antigen-1 (SCA1) and plasmalemmalstem cell factor receptor (SCFR),4 but not plasmalemmal markers of lineage-committed hematopoietic cells (Lin − ).5 This stem cell subset is hence defined as Lin − , SCA1 + , SCFR (KIT) + (LSK) cells.
2.1.2 Hemangioblast
Both hematopoietic and endothelial cells arise from a mesoderm-derived VEGFR2 + common precursor, the so-calledhemangioblast.6 Hematopoietic progenitor cells arise from these bipotential precursors via a subset of early endothelial cells, i.e., differentiated endothelial cells that have a hematopoietic potential and form thehemogenic endothelium [49].
TIE2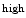 , SCFR + hemogenic endothelium is transiently generated. Hemangioblasts isolated from differentiated mouse embryonic stem cells in culture generate tightly adherent structures (stage 1) and then non-adherent round cells that proliferate to generate a mature blast colony (stage 2) [50].7
, SCFR + hemogenic endothelium is transiently generated. Hemangioblasts isolated from differentiated mouse embryonic stem cells in culture generate tightly adherent structures (stage 1) and then non-adherent round cells that proliferate to generate a mature blast colony (stage 2) [50].7
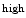 , SCFR + hemogenic endothelium is transiently generated. Hemangioblasts isolated from differentiated mouse embryonic stem cells in culture generate tightly adherent structures (stage 1) and then non-adherent round cells that proliferate to generate a mature blast colony (stage 2) [50].7
, SCFR + hemogenic endothelium is transiently generated. Hemangioblasts isolated from differentiated mouse embryonic stem cells in culture generate tightly adherent structures (stage 1) and then non-adherent round cells that proliferate to generate a mature blast colony (stage 2) [50].7 During embryogenesis, SCA1 + , SCFR + (CD117 + ), CD41 + (Itgα2B + ) hematopoietic stem cells emerge directly from the aortic floor into the dorsal aortic lumen, at least in some species, following egress of Runx1 + endothelial cells from the aortic ventral wall into the subaortic space [51, 52]. Cadherin-5 + (or vascular endothelial [VE]-cadherin) endothelial precursors or hemogenic endothelial cells transiently possess the ability to give rise to multipotent hematopoietic stem and progenitor cells during vertebrate development (endothelial–hematopoietic transition) [53]. These cells afterward enter the blood circulation to colonize and differentiate in hematopoietic organs.
2.2 Biological Models of Hematopoiesis
Hematopoiesis relies on classical and alternative pathways. Long-term hematopoietic stem cells give rise to short-term hematopoietic stem cells that have a transient ability to self-renew and differentiate into multipotent progenitors [54].
The latter lack the capacity of self-renewal, but retain multipotency, creating a series of intermediate progenitors, such as common lymphoid and myeloid progenitors that further differentiate into lymphoid and granulocyte–macrophage progenitors, respectively.
Lymphoid-primed multipotent progenitors can develop lymphoid and myeloid progeny, but not erythroid and megakaryocytic cells.
Granulocyte–macrophage progenitors generate myelomonocytic and megakaryo- cytic–erythrocyte progenitors. However, the separation between the myeloid and megakaryocytic–erythroid cell lineages can occur before the development of common myeloid progenitors.
Myelo-erythroid progenitors include pregranulocyte–macrophage precursors that give rise to granulocyte–macrophage progenitors, whereas prepremegakaryocytic–erythroid precursors yield preCFUe (CFU erythroid), CFUe, and megakaryocytic–platelet (MKP) compartments.
2.2.1 Arborescence Models of Hematopoiesis
An arborescence model of hematopoiesis assumes branches for the differentiation of blood cells [55]. This model states that: (1) megakaryocytes as well as erythroid and myeloid cells from a multipotent progenitor (MPP), lymphoid-primed multipotent progenitor (LMPP), early lymphoid progenitor (ELP), or common myeloid progenitor (CMP); and (2) natural killer cells and B and T lymphocytes from a common lymphoid progenitor (CLP).
Another tree-like model supposes that: (1) a common myeloid–lymphoid progenitor (CMLP) gives rise to (1.1) a myeloid–T-cell progenitor (MTP) that differentiates into myeloid and T lymphocytes and (1.2) myeloid–B-cell progenitor (MBP) that gives rise to myeloid and B lymphocytes; and (2) a common myeloid progenitor generates myeloid cells and a myeloid–erythroid progenitor (MEP), a source of erythroid cells and megakaryocytes.
A third model proposes that erythroid potential is lost early from a common myeloid progenitor that differentiates into granulocyte–monocyte progenitor (GMP) with myeloid potential and myeloid–erythroid progenitor, whereas a common lymphoid progenitor leads to lymphoid cells.
2.2.1.1 Arborescence Model Drawbacks
Lymphoid progenitors can have a myeloid potential and differentiate into myeloid cells (macrophages and dendritic cells). Therefore, models of early segregation of lymphoid and myeloid differentiation axes are not validated by observations.
2.2.2 Lymphoid Progenitors
Most Lin − , SCA1 + , SCFR + , FLT3 + lymphoid-primed multipotent progenitors lack erythroid and megakaryocytic potential. Several lymphoid progenitors that are able to generate B and T lymphocytes and natural killer cells include: (1) Lin − , SCA1 + , SCFR + , FLT3high, VCAM1 − lymphoid-primed multipotent progenitors; (2) SCFRhigh early lymphoid progenitors that express recombination-activating gene RAG1;8 and (3) SCFR − , PTPRc + , CLP2 (type-2 common lymphoid progenitor) progenitors that express the gene encoding the preT-cell antigen receptor.9
Early progenitors with lymphoid and myeloid potential (EPLM), lymphoid-primed multipotent progenitors (LMPP), and thymic progenitors that have both lymphoid and myeloid potential receive many signals that govern their fate. This destiny depends on the type of growth factors as well as strength and duration of signaling.
Lymphoid-primed multipotent progenitors have both lymphoid and myeloid potential, with high granulocytic, monocytic, and lymphoid potential, but low potential for megakaryocyte or erythroid development [55]. However, an LMPP subpopulation that expresses the thrombopoietin receptor has a megakaryocytic and erthyroid potential.
The earliest thymic progenitors retain myeloid potential, hence can contribute to thymic granulocyte and macrophage populations, but not B cells. Early T-cell progenitors, or CD4 − , CD8 − double-negative cells (DN), comprise:
2.
SCFRhigh, CD44 + , CD25 − DN1 cells;
3.
SCFRhigh, CD44 + , CD25 + DN2 cells;
4.
SCFR ± , CD44 − , CD25 + DN3 cells; and
5.
SCFR − , CD44 − , CD25 − DN4 cells.
Notch-1 is required for T-cell development. When it is lacking in the thymus, thymocyte progenitors bifurcate toward B-cell development.
Early thymocyte progenitors (ETP) can be subdivided according to expression ofstem cell protein Tyr kinase-1 (STK1) receptor11 and chemokine CCR9 receptor. Only STK1 + or CCR9 + thymus-settling progenitors have B-cell potential [55]. Double-negative DN1 and DN2 cells are not committed to T-cell development, but give rise to NK and myeloid cells.
The pairwise relationship model of hematopoiesis [55] does not take into account branching patterns that determine preferred route for a given cell fate. It displays in a circle blood cell sectors (megakaryocyte, erythrocyte, basophil or mastocyte, eosinophil, neutrophil, monocyte, dendritic cell,12 and B, NK, and T cell) and arcs inside the disk assigned for each progenitor and precursor with overlaps (as a final cell fate can be reached via several types of intermediate progenitors) or involved transcription factors in hematopoietic stem cells.
2.3 Stem Cell Niches
The embryonic origin of adult hematopoietic stem cells (multipotent progenitors) is the aorta–gonad–mesonephros region. Hematopoietic stem cells subsequently colonize fetal and adult hematopoietic organs, i.e., fetal liver and spleen on the one hand and, in adults, the bone marrow as well as spleen and liver during hematopoietic stress on the other. Colony-forming units in the spleen reconstitute transiently multipotent progenitors.
2.3.1 Types of Bone-Marrow Niches
Different niche types for hematopoietic stem cells exist: (1) osteoblastic and (2) vascular bone marrow HSC niches. In adults, hematopoietic stem cells have their own bone marrow microenvironment,13 close to theendosteal14 surface of bone marrow cavities in trabecular regions of long bones, whereas more differentiated hematopoietic progenitors are mainly located in the central bone marrow region [56]. The endosteal niche is characterized by a sinusoidal endothelium. Changes in the HSC niche do not allow appropriate HSC maintenance in vivo.
In addition to osteoblastic niches, the vascular bone marrow HSC niche demarcated by bone marrow sinusoidalendothelial cells is a second specialized HSC microenvironment in the bone marrow, with a large quantity of SLAMF1 + hematopoietic stem cells attached to the fenestrated endothelium of bone marrow sinusoids. Endothelial cells of bone marrow sinusoids yield a cellular platform for the differentiation of lineage-committed progenitors such as megakaryocytic progenitor cells (CFU megakaryocyte).
Bone marrow vascular and endosteal niches cooperate to control HSC quiescence and self-renewal. A small number of hematopoietic stem cells is constantly released into the blood circulation. Endothelial cells of bone marrow sinusoids differ from endothelial cells of the microvasculature of any other organ. These endothelial cells express cytokines such as chemokine CXCL12,15 like osteoblasts, and adhesion molecules for HSC mobilization, homing,16 and lodging in endosteal bone marrow HSC niches [56]. SLAMF1 + hematopoietic stem cells close to sinusoids may monitor the concentration of blood elements.
Hematopoietic stem cell niches regulate the stem cell maintenance in cooperation with endosteal cells (osteoblasts and osteoclasts) at the interface between bone and bone marrow, as well as vascular and perivascular cells around bone marrow sinusoids. The specific microenvironment promotes stem cell survival and self-renewal and regulates cell differentiation and migration according to the body’s need. Endosteal, vascular, and perivascular cells cooperate and contribute to the HSC maintenance, expansion, and location. Homeostasis of hematopoietic stem cells requires many factors (Table 2.2) [57].
Table 2.2
Factors of the maintenance of hematopoietic stem cells (Sources: [57, 58]; MPL: myeloproliferative leukemia virus proto-oncogene product). Stromal and parenchymal cells of the bone marrow (mesenchymal progenitors, osteoblasts, fibroblasts, adipocytes, and reticular and endothelial cells), endosteal cells (undifferentiated bone-lining cells, bone-forming osteoblasts, and bone-resorbing osteoclasts), and vascular and perivascular cells of bone marrow sinusoids secrete factors that promote HSC maintenance. Osteoblasts secrete angiopoietin, thrombopoietin, and CXC-chemokine ligand-12 (CXCL12). Angiopoietin (also secreted by megakaryocytes and perivascular cells in the bone marrow) and thrombopoietin (supplied by blood or produced by stromal cells) promote HSC quiescence; CXCL12 (also secreted by reticular and perivascular cells) regulates HSC migration (CXCR4: CXC-chemokine receptor-4). Alternative splicing creates many epican variants (a.k.a. CD44 and homing cell adhesion molecule [HCAM]), especially the smallest epicanS (or hematopoietic CD44h) that is strongly expressed on hematopoietic cells and long epicanL (CD44v). Due to diversity of extracellular domain, epican interacts with numerous ligands (hyaluronate, fibronectin, collagen-1 and -4, serglycin, osteopontin, etc.).
Factor | Receptor, partner |
|---|---|
Angiopoietin | TIE1/2 |
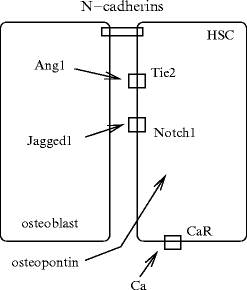
N-cadherin | Ca2 + -dependent N-cadherin receptor |
Calcium | Ca2 + -sensing receptor |
CXCL12 | CXCR4 |
Osteopontin | Epican (CD44), αVβ3-integrin |
Prostaglandin-E2 | Cyclooxygenase and synthase |
Sonic Hedgehog | Patched |
Stem cell factor | Stem cell factor receptor (KIT) |
Thrombopoietin | Thrombopoietin receptor (MPL) |
2.3.2 Cells of Bone Marrow Niches
Osteopontin + , N-cadherin + osteoblasts that line the endosteal surface of trabecular bone,bone marrow sinusoid endothelial cells, CXCL12-secreting stromal cells, adipocytes, andmacrophages constitute functional niches of hematopoietic stem cells in adult bone marrow.
The adipocyte number is inversely correlated with the hematopoietic activity in the niche. Density of hematopoietic stem cells and progenitors decays in the adipocyte-rich bone marrow. Bone marrow adipocytes operate as inhibitors of hematopoiesis [59].
On the other hand, in vitro, undifferentiated stromal cells and preadipocytes support hematopoiesis, as they produce growth factors, such as granulocyte–macrophage (CSF2) and granulocyte (CSF3) colony-stimulating factor. Moreover, adipocytes secreteneuropillin-1,lipocalin-2,18adiponectin, andtumor-necrosis factor-α that can preclude hematopoietic cell proliferation. Although adipocytes prevent expansion of hematopoietic progenitors, they preserve hematopoietic stem cell pool.
2.3.3 Crosstalk between Niche-Resident Cells
Molecular crosstalk between hematopoietic stem cells and other cellular constituents of endosteal niches regulate the balance between HSC self-renewal and differentiation. Cellular adhesions between stem cells and stromal cells and/or the extracellular matrix anchor stem cells within the niche and regulate stem cell behavior. Whereas β1– and β7-integrins are not necessary to maintain retention of adult hematopoietic precursors in the bone marrow, other agents, such as membrane-boundstem cell factor19 and its receptorSCFR, as well as CXCR4 can mediate their sequestration in the niche [62].
Osteoblasts secrete multiple cytokines that promote the proliferation of hematopoietic cells. Specialized spindle-shapedN-cadherin-expressing osteoblasts of the endosteum directly contact hematopoietic cells via N-cadherin.20 Many stromal cell lines of endosteal bone surfaces that are involved in bone modeling, are required in HSC maintenance. The endosteal niche can contain quiescent and self-renewing hematopoietic stem cells.
Osteopontin that prevents the proliferation of hematopoietic stem cells may maintain HSC quiescence (Fig. 2.2). Angiopoietin-1 at the osteoblast surface interacts with its receptorTyr kinase with Ig and EGF homology domains TIE2 on stem cells to maintain stem cell quiescence in the niche.
Certain situations such as inflammation trigger the activity of osteoclasts along the stem cell niche, secretion of enzymes (cathepsin-G, elastase, MMP9, etc.) and cytokines (interleukin-8), and mobilization of progenitors from the bone marrow to the circulation [63]. Osteoclasts require TNFSF11 from osteoblasts for proliferation and bone resorption. Activating osteoclasts via either TNFSF11 or other stimuli leads to emigration of hematopoietic stem cells into the circulation. On the other hand, increased osteoblast activity via parathyroid hormone receptor or inactivation of thebone morphogenic protein receptor-1A causes a proliferation of hematopoietic stem cells.
Endothelial cells in vascular niche signal to hematopoietic stem and progenitor cells, as they release specific paracrine growth factors, the so-called angiocrine factors.Protein kinase-B activation in endothelial cells via TOR, but not the FoxO pathway, upregulates specific angiocrine factors that support self-renewal and expansion of CD34 − , STK1 − , Lin − , SCA1 + , SCFR + hematopoietic stem and progenitor cells (LSK cells), hence the long-term hematopoietic stem cell repopulation [929]. On the other hand, coactivation in endothelial cells of PKB andextracellular signal-regulated kinases ERK1 and ERK2 favors maintenance and lineage-specific differentiation of hematopoietic stem and progenitor cells. Selective activation of PKB1 in endothelial cells of adult mice increased the number of colony-forming units in the spleen and CD34 − , STK1 − , LSK long-term hematopoietic stem and progenitor cells in the bone marrow [929].
2.4 Regulation of Hematopoiesis
Hematopoietic stem cells express the plasmalemmal hematopoietic progenitor antigen cluster of differentiation CD34 (CD34 + cells), a glycoprotein (sialomucin protein) that acts as a cell adhesion molecule for attachment of stem cells to the extracellular matrix or stromal cells.21 Expression of CD34 allows activated early progenitors to be distinguished from quiescent cells. CD34-related molecules — podocalyxin and podocalyxin-like molecule-2 (or endoglycan) — are also expressed on stem and early progenitor cells [65, 66] (Table 2.3).22 Proteins of the CD34 family enhance proliferation, but repress differentiation of stem and progenitor cells, partly due to defects in adhesion and migration [66]. Both CD34 and podocalyxin improve migration of hematopoietic cells. Members of the CD34 family promote or block cell adhesion according to their expression level. They facilitate adhesion of lymphocytes to specialized vascular endothelia in lymphoid tissues such as that of high endothelial venules. On the other hand, their negatively charged, glycosylated extracellular domain can impede intercellular aggregation [66].
Table 2.3
Distribution of CD34 family members (Source: [66]; + : expression; − : no expression). Cell-surface marker CD34 enables the identification of hematopoietic stem cells and progenitors. CD34-related proteins include podocalyxin and endoglycan.
Distribution | CD34 | Podocalyxin | Endoglycan |
|---|---|---|---|
Multipotent precursors | + | + | + |
Eosinophils | + | − | − |
Erythrocytes | − | Anemia | − |
T lymphocytes | − | − | Thymocytes |
B lymphocytes | − | − | TLR-activated B cells |
Macrophages | − | − | − |
Mastocytes | + | − | − |
Thrombocytes | − | + | − |
Muscle satellite cells | + | − | − |
Hair follicle stem cells | + | − | − |
Vascular endothelia | + | + | + |
Smooth myocyte | − | − | + |
Fibrocytes | + | − | − |
Mesothelia | − | + | − |
Neurons | Weak | + | Some |
Podocytes | − | + | Weak |
Numerous substances regulate hematopoiesis using a specific set of transcription factors. They include morphogens and growth factors (Table 2.4) that prime signaling cascades with given effectors down to transcription factors for gene expression. In addition, hematopoietic stem cells are mobilized by the sympathetic nervous system.
Table 2.4
Regulators of hematopoiesis. Canonical Wnt and Jagged-1–Notch-1 signaling promote HSC survival and proliferation particularly after damage. Insulin-like growth factor binding protein-3 (IGFBP3) favors progenitor recruitment to hypoxic sites (CSF: colony-stimulating factor; CSFR: colony-stimulating factor receptor; LRP: LDL receptor-related protein).
Factor | Receptor and |
|---|---|
partners | |
Erythropoietin | EpoR |
Granulocyte CSF (CSF3) | CSF3R; Ras |
Granulocyte–macrophage CSF (CSF2) | CSF2R; Ras |
IGF1 | IGF1R; IGFBP3 |
Interleukins | ILR |
Jagged-1 | Notch |
Macrophage CSF (CSF1) | CSF1R; Ras |
Sonic Hedgehog | Patched |
Stem cell factor | SCFR |
Thrombopoietin | TpoR |
Tumor-necrosis factor-α | TNFR |
Wnt | Frizzled, LRP; Disheveled, |
β-catenin | |
STAT | JaK |
Under normal conditions,bone morphogenetic proteins,osteopontin,23Wnt inhibitors secreted Frizzled-related proteins sFRP1, and non-canonical Wnts are expressed in the endosteal zone to yield an inhibitory microenvironment to favor the pool of long-term quiescent hematopoietic stem cells [40].
On the other hand,fibroblast growth factors, canonicalWnt signals, and CXCL12 expressed from endothelial, megakaryocytic, and CXCL12high reticular cells stimulate the active subpopulation of hematopoietic stem cells in the central zone.
2.4.1 Transcription Factors of Hematopoiesis
Transcriptional regulation controls and maintains the hematopoietic stem cell pool in the bone marrow as well as lineage commitment. Transcription factors of the E protein family (TcF3, TcF4, and TcF12), i.e., bHLHb19 to bHLHb21 (Table 2.5), andinhibitors of DNA-binding (ID), i.e., bHLHb24 to bHLHb27, which are synthesized in hematopoietic cells, regulate many developmental processes.
Table 2.5
Subclass-B basic helix–loop–helix proteins that belong to E/E2A protein and inhibitor of DNA binding (ID) families. Transcription factors of the E protein family can heterodimerize with subclass-A basic helix–loop–helix protein bHLHa17 (a.k.a. T-cell acute lymphocytic leukemia protein-1 [TAL1] and stem cell leukemia factor [SCL]) and bHLHa18 (a.k.a. lymphoblastic leukemia-derived sequence-1 [LyL1]).
bHLHb type | Main alias | Other aliases |
|---|---|---|
E2A transcription factors | ||
bHLHb19 | TcF4 | E2-2, TcF7L2, ITF2, SEF2 |
bHLHb20 | TcF12 | HEB, HTF4 |
bHLHb21 | TcFE2α/TcF3 | E2A, ITF1 |
bHLHb21E12 | TcFE2a | E12 |
bHLHb21E47 | TcF3 | E47 |
E protein partners (heterodimers) | ||
bHLHa17 | TAL1 | SCL, TCL5 |
bHLHa18 | LyL1 | |
Inhibitors of DNA binding | ||
bHLHb24 | ID1 | |
bHLHb25 | ID3 | |
bHLHb26 | ID2 | |
bHLHb27 | ID4 | |
Progenitor fate depends on coordinated activities of multiple transcription factors, such asActivator protein AP1,CCAAT/enhancer-binding protein C/EBPα,early B-cell factor EBF1,erythroid differentiation-associated gene product (EDAG), or hemogen,24erythroid Krüppel-like factor (KLF1 or EKLF), members of the GATA family, zinc finger protein multitype ZFPM1,25 andPaired box protein Pax5.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree