Heat Failure in Cardiac Tamponade, Constrictive Pericarditis, and Restrictive Cardiomyopathy
Ralph Shabetai
Constrictive pericarditis and cardiac tamponade both manifest as specific forms of heart failure with preserved systolic function. While cardiac tamponade is usually managed by intensivists or interventional cardiologists, the syndrome of constrictive pericarditis shares many features of right heart failure of other etiologies, especially restrictive cardiomy-opathy, and therefore is particularly relevant to heart failure, particularly diastolic heart failure. I have therefore emphasized constrictive pericarditis and its differential diagnosis from restrictive cardiomyopathy in this chapter.
Cardiac Tamponade
Definition
The definition of cardiac tamponade is pericardial fluid under increased pressure that compresses the heart, thereby impeding diastolic filling, and does not permit change in total cardiac volume. Systolic function of the left ventricle as judged by its ejection fraction (LVEF) is not impaired, and often is increased because of the increase in adrenergic and sympathetic tone that accompanies cardiac tamponade. Cardiac tamponade is thus an important but uncommon cause of diastolic heart failure.
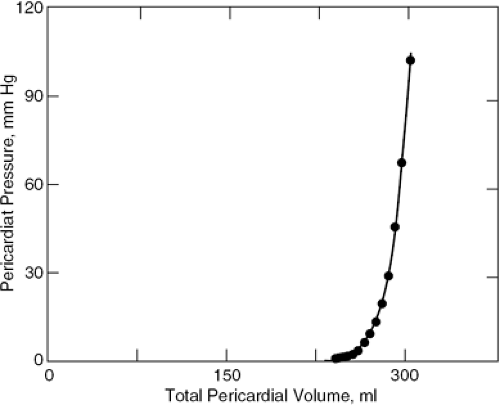
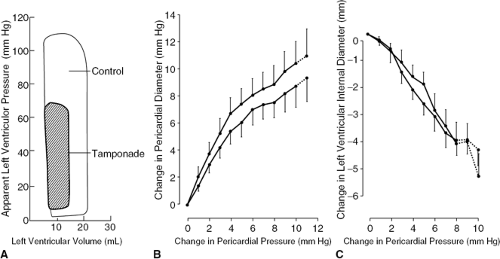
Almost every pericardial disease may be effusive and any effusion may accumulate faster than it can be absorbed and therefore may stretch the pericardium. When the pericardium is stretched it becomes increasingly stiff and eventually inextensible because the pressure volume and stress-strain curves of normal pericardium are J-shaped (Fig. 22-1). Once the pericardium has been tightly stretched, the volume of the pericardial cavity becomes fixed; this invariant pericardial volume underlies much of the pathophysiology of cardiac tamponade. Nevertheless, studies of diastolic heart failure or heart failure with preserved systolic function have shown that in these patients analysis of myocardial function discloses impairment of clinically insignificant myocardial function. This may be true for some cases of tamponade as well, but the issue has not been investigated and, in any case, is not sufficient to contribute to heart failure.
Etiology
Chief offenders include pericardial injury, occurring either at the same time as tamponade, or earlier, as in a postperi-cardial injury syndrome. Other causes include inflammation of any cause, neoplastic pericardial disease, acute viral or idiopathic pericarditis, tuberculous pericarditis, and mediastinal radiation. Acquired immunodeficiency syndrome (AIDS) frequently causes pericardial effusion but tamponade is uncommon unless there is a superimposed infection to which these patients are liable. The effusion is often small but is associated with poor prognosis unrelated to hemodynamics (1).
By the time patients with cardiac tamponade seek medical care their tamponade is acute, but this event may occur either immediately after a penetrating pericardial injury or be delayed for weeks or more in the case of trauma that may be blunt, as in steering wheel accidents or a blow from a fast, hard ball. Cardiac tamponade should be considered in any patient with unexplained hemodynamic deterioration after cardiac surgery. This event may occur hours after the operation has been concluded but may be delayed, sometimes until after the patient has been discharged home.
Pathophysiology
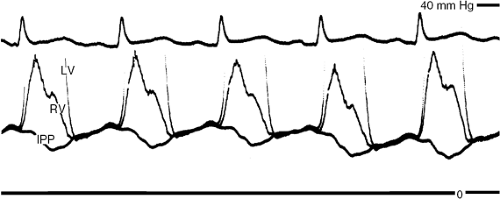
The clinical and laboratory findings are explained by the pathophysiology. Once the pericardium has been tightly stretched, it becomes inextensible; therefore, total pericardial volume cannot vary. The consequence is that if one cardiac chamber or side of the heart increases in volume, the opposite one must decrease. Attention in this regard has been focused on the ventricles and is termed ventricular interaction or interdependence. The clinical manifestation of increased ventricular interaction is pulsus paradoxus (2), a greater than normal decline in systemic arterial systolic and pulse pressures, the latter reflecting decreased stroke volume. Diastolic pressure variation remains normal. While ventricular interaction is the predominant cause of pulsus paradoxus in tamponade, other mechanisms, described elsewhere (2), contribute.
Because of the multiplicity of mechanisms, pulsus paradoxus in the two circuits is almost, but not always, precisely 180 degrees out of phase, such that peak systolic pressure of one ventricle is highest when that of the opposite ventricle is lowest. This phase relationship is known as ventricular discordance. It is another manifestation of ventricular interdependence and, as we shall see later, also occurs in constrictive pericarditis where it is of critical importance in the differential diagnosis from restrictive cardiomyopathy, in which it is absent. The major hemody-namic signs of cardiac tamponade are a pulsus paradoxus in both the systemic and pulmonary circulation, and a large increase in the amount that inspiration decreases filling of the left ventricle and increases that of the right. These abnormalities are readily detected by cardiac catheterization or echo-Doppler cardiography (3).
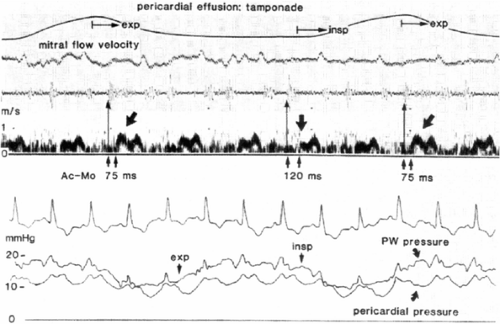
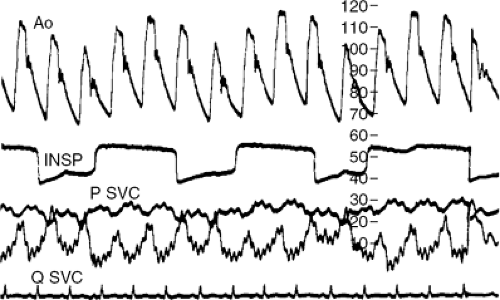
Another fundamental pathophysiological feature is that, in spite of even severe tamponade, the inspiratory decline in intrathoracic pressure is transmitted (although not completely) to the pericardium and heart; therefore, systemic venous return increases, much as it does in normal subjects (2,4,5). Because the pericardial volume cannot increase, the consequent increase in right ventricular volume is accomplished entirely by bulging the septum from right to left, encroaching on left ventricular volume. Cardiac volume is redistributed, not changed. The drop in central venous pressure with inspiration has the same mechanism as in normal subjects but is greater because of ventricular interaction. It can be detected at the bedside by inspecting the jugular pulse; also, the reciprocal changes in left and right ventricular dimensions and areas are readily seen on M-mode and two-dimensional echocardiograms. Because inspiration lowers pressure in the pulmonary veins that lie in the thorax but outside the pericardium, whereas the left ventricle lies entirely within the pericardium, inspiration lowers pulmonary wedge pressure more than left ventricular diastolic pressure (Fig. 22-2). This reduction in the pressure gradient for left ventricular filling makes a further contribution to diminished left ventricular volume during inspiration.
Raised pericardial pressure compresses the thin-walled atria (6) and right ventricle (7) but usually the left ventricle is hardly, if at all, directly compressed. Thus, the chief mechanism that reduces left ventricular volume is ventricular interaction, but diminished pulmonary venous return by the compressed right ventricle makes a contribution. Echocardiography clearly demonstrates compression of the right atrium (8) and collapse of the right ventricular cavity in early diastole (9,10), a time when its pressure is minimal. The pathophysiology and, hence, the clinical features of tamponade in most medical patients in whom effusion develops slowly differ importantly from those of surgical patients in whom effusion is often virtually instantaneous (11).
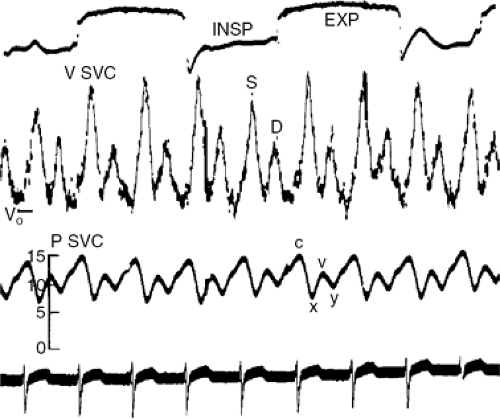
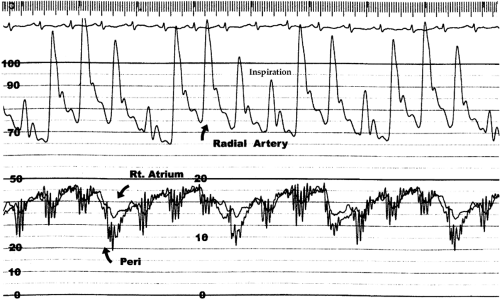
Total cardiac volume is minimal during ventricular ejection and is maximal in late diastole; therefore, tamponade is slightly less severe during the ejection period and is most severe in late diastole. Venous return, instead of taking place both in the ejection period (noted clinically as the x descent of venous pressure) and immediately after the atrio-ventricular valves open (noted by the y descent) (Fig. 22-3), is confined to the period of ejection (12). The jugular pulse, therefore, shows a prominent x descent but no y descent (Fig. 22-4).
Cardiac tamponade may be acute or chronic and causes, characteristic clinical and hemodynamic signs. Atypical variants also occur, the most important of which are low-pressure tamponade, sometimes referred to as occult tamponade, and regional tamponade in which the fluid is loculated and therefore does not exert pressure circumfer-entially, but over a localized area of the heart. In some patients, cardiac tamponade and constrictive pericarditis occur together, a syndrome known as effusive-constrictive pericarditis. These variants will be discussed later in the chapter.
The hemodynamics of tamponade, owing to rupture of an aortic aneurysm of the heart or laceration of a coronary vessel or bypass graft, differ from those of postpericardial injury and other causes of tamponade as a result of the extreme rapidity of the effusion, because the pericardium is stretched in inverse proportion to the rate of effusion. When effusion is virtually instantaneous, pericardial stretch is minimal; therefore, the effusion cannot be large but pericardial pressure is extremely high and causes rapidly progressive shock (Figs. 22-5 and 22-6). An effusion that accumulates more slowly progressively stretches
the pericardium, causing it to remodel with increased compliance.
the pericardium, causing it to remodel with increased compliance.
Cases seen in the practice of medicine are usually less acute [medical tamponade, (11)]; therefore, the effusion is considerably larger, causing radiological cardiomegaly, and pericardial pressure elevation is less severe but high enough to cause clinically significant cardiac tamponade (Fig. 22-7).
Typical Medical Tamponade
Clinical Examination
The history often discloses factors mentioned under Etiology, previously discussed. The predominant symptom is dyspnea, often with orthopnea, and many patients report fullness in the chest. In the more severe cases, the patients note lightheadedness or presyncope that eventually progresses to cardiogenic shock and death, unless treated.
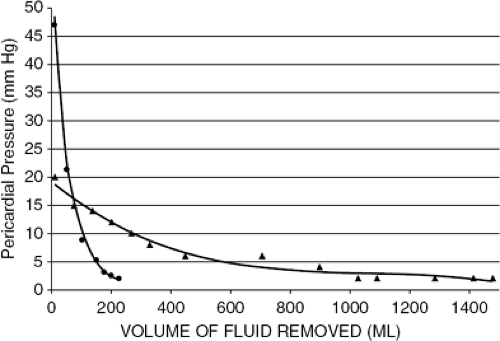 Figure 22-5 Pericardial pressure-volume curve records from a case of hyperacute cardiac tamponade (illustrated in Fig. 22-6) and from a case of cardiac tamponade complicating acute pericarditis. The curve from hyperacute tamponade shows the small volume of the effusion and a rapid decline in pericardial pressure when a small volume of fluid was withdrawn. (Reproduced from Shabetai R. Cardiac tamponade. In: Shabetai R. The Pericardium. Norwell, MA: Kluwer Academic Publishers; 2003:121–166 .) |
On examination, the patient is in distress. In milder cases, the systemic arterial blood pressure is normal but, with increasing severity, falls progressively. In severe cases, signs of low cardiac output and reduced perfusion are present.
Sinus tachycardia is seen in almost all patients, allowing for at least partial maintenance of cardiac output. One exception is subacute tamponade, due to the pericardial effusion associated with hypothyroidism, in which the underlying disease tends to produce bradycardia. Patients with early tamponade have elevated jugular pressure but are fully neurohormonally compensated and therefore do not have tachycardia. A pericardial rub may be heard in patients with inflammatory pericarditis (13).
The jugular venous pressure is almost always elevated. The x descent (the first inward deflection of the internal jugular pulse during systole due to atrial relaxation and downward displacement of the tricuspid valve with ventricular ejection) is preserved. However, the y descent (the second inward deflection of the internal jugular pulse due to diastolic inflow of blood into the right ventricle) is attenuated or absent (12) because of the limited or absent late diastolic filling of the right ventricle.
Pulsus paradoxus, defined as decrease in systolic blood pressure exceeding 10 mm Hg on inspiration, is a common finding in moderate to severe tamponade. To measure pulsus paradoxus, a sphygmomanometer is employed for blood pressure measurement in the standard fashion except that the cuff is deflated more slowly than usual. During deflation, the first Korotkoff sounds are audible only during expiration but, with further deflation, Korotkoff sounds are heard throughout the respiratory cycle. The difference between the systolic pressure, at which the first Korotkoff
sounds are heard during expiration, and the pressure at which they are heard throughout the respiratory cycle quantifies pulsus paradoxus.
sounds are heard during expiration, and the pressure at which they are heard throughout the respiratory cycle quantifies pulsus paradoxus.
In a number of conditions pulsus paradoxus is usually absent despite cardiac tamponade. Pulsus paradoxus occurs in the absence of pre-existing heart disease because the diastolic pressure in both ventricles is identical, being equal to pericardial pressure. Compliance of the two ventricles is thus equalized. Pre-existing heart disease prevents this equalization of ventricular diastolic compliance and pulsus paradoxus is therefore absent. Common examples include hypertension and chronic renal disease, in which the left ventricle is hypertophied and therefore is stiffer than normal (14). Right ventricular hypertrophy prevents the pulsus paradoxus of tamponade in the same manner (15). The volume of a large left-to-right shunt through an atrial septal defect (ASD) does not vary with the respiratory cycle because it has no extrathoracic component and greatly exceeds systemic venous return; therefore, pulsus paradoxus is absent (16). Because the volume of severe aortic regurgitation is constant throughout respiration, pulsus paradoxus is absent in this condition, too. In some of these cases, a left ventricular diastolic pressure higher than pericardial pressure is an additional factor preventing pulsus paradoxus. Rupture of an aortic aneurysm into the pericardium is an important example of acute tamponade in which pulsus paradoxus may be absent. Tamponade may stop hemorrhage from the ruptured myocardium, allowing time for surgical repair. Pericardiocentesis should not be performed but, if it is deemed life-saving, the volume of blood aspirated should be kept to the minimal Possible amount.
Electrocardiogram
The electrocardiogram (ECG) changes usually are not specific. Occasionally, ST segment or PR depression indicating acute pericarditis is present. Sinus tachycardia is usual.
Cardiac tamponade is a sensitive but not specific cause of low voltage (17). When the effusion is massive, electrical alternans may ensue (18,19) but lacks specificity except when it includes alternation of the P and T waves as well as the QRS complex. The combination of pericardial effusion and low voltage should alert physicians and medical staff to the possibility of cardiac tamponade.
Chest Radiogram
The cardio-pericardial silhouette is increased without selective enlargement of any specific chamber. The lung fields are less congested than in cases of heart failure of other etiology, except when accompanied by severe tricus-pid regurgitation. An unexplained increase in the silhouette and hemodynamic deterioration also suggest cardiac tamponade.
Echocardiogram
Pericardial effusion is a sine qua non for cardiac tamponade. In most cases of medical tamponade the effusion is substantial. Echocardiography is the optimal means of establishing the absence or presence of pericardial effusion and is indicated whenever pericardial effusion or cardiac tamponade is suspected; this indication has been endorsed by several published guidelines (20).
Echocardiography not only provides evidence of the size, distribution, and nature of the effusion but also of the likelihood of the patient having tamponade. Right atrial compression (8) and early diastolic ventricular collapse (9) are readily identified by this means. M-mode shows reciprocal respiratory variation of the magnitude of the left and right ventricular diastolic dimensions; the timing of ventricular collapse can be shown to be in diastole by simultaneous imaging of the open mitral or closed aortic valve. Increased diameter of the inferior vena cava and absent or less than 50% collapse with inspiration or a sniff are nicely documented by M-mode (21).
Two-dimensional Doppler echocardiography furnishes valuable additional information. Compression of the right heart chambers and, less commonly, of the left (22), especially the atrium (23), is identified in several image planes. Just as patients with cardiac tamponade who also have heart disease may not show pulsus paradoxus (14,16), likewise coexisting heart disease may prevent the expected compression of the right atrium and ventricle (24). Similarly, severe right heart failure may greatly elevate right ventricular diastolic and right atrial pressures, such that if the patient develops cardiac tamponade, the right heart diastolic pressures exceed pericardial pressure and, thus, collapse of the right heart chambers is prevented.
Like the inferior vena cava, the hepatic veins are engorged. Thus, the study provides ample proof of plethora in the majority of cases of at least moderate severity. Low-pressure tamponade is a significant exception to this rule (25).
Doppler interrogation of the atrio-ventricular orifices provides clear evidence of ventricular interdependence in that respiratory variation is greatly increased. During inspiration, the mitral E wave normally declines by up to 10%, but in tamponade may fall by as much as 45% (3,26). Reciprocal and somewhat larger changes are seen in the
tricuspid E wave. Pulsus paradoxus is documented by decreased aortic stroke volume during inspiration.
tricuspid E wave. Pulsus paradoxus is documented by decreased aortic stroke volume during inspiration.
Cardiac Catheterization
From the foregoing, it is apparent that the diagnosis can be readily made without cardiac catheterization, and usually is; therefore, technical details are not given here but have been published elsewhere (27). It is, however, relevant to re-emphasize here that accurate calibration of pressure transducers is critical for demonstrating diastolic pressure equalization. Relevant pressures should be recorded simultaneously, preferably superimposed and at high gain. Left-and right-sided pressures should also be recorded at low gain so as to visualize the phase relation of peak systolic pressures in the systemic and pulmonary circulation throughout several respiratory cycles. Finally, in this regard a respirometer tracing is very useful but, if one is not available, pulmonary wedge pressure should be recorded with other pressures of interest as a marker of the phase of respiration.
An example of the findings from a typical case of cardiac tamponade in a patient with viral or idiopathic pericarditis is shown in Figure 22-7. Even coexisting heart disease is easily recognized and evaluated noninvasively. However, right heart catheterization should, in my opinion, be included with pericardiocentesis to ascertain that pericardial pressure has been restored to near normal and to exclude effusive-constrictive pericarditis, in which pericardiocentesis normalizes pericardial (but not right atrial and pulmonary) wedge pressures (28,29).
Left ventriculography is no longer performed for the diagnosis or evaluation of cardiac tamponade, but construction of pressure-volume loops (usually using a volume-sensing catheter) is a useful research tool. A beautifulexample of a left ventricular pressure-volume loop using opaque contrast medium on a canine model of tamponade, showing elevated diastolic pressure, hypotension, and low stroke volume, was published in 1968 (30) (Fig. 22-8).
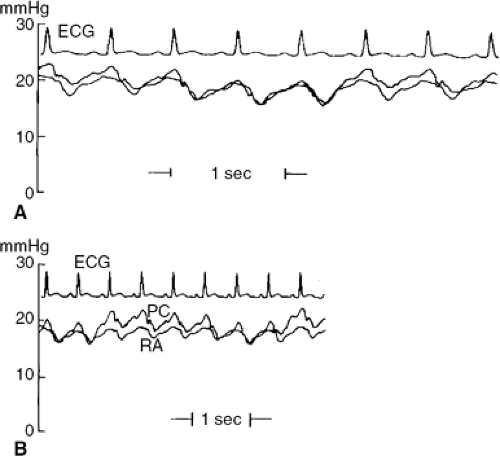
Before a significant volume of fluid has been aspirated, the right atrial, pulmonary wedge, pericardial right ventricular, and pulmonary arterial diastolic pressures are all elevated and are just about equal to each other. In typical medical tamponade (11), the pressures are usually in the range of 15 to 25 mm Hg. The right atrial (and central venous) pressure shows a normal fall with inspiration, and the normal bimodal waveform with x and y descents is replaced by a waveform in which the y descent is replaced by a progressive upslope leading to end-diastole (12) (Fig. 22-9).
Treatment
The appropriate treatment depends on the severity of tamponade that varies from subclinical, through mild and moderate, to severe (14,26). Mild tamponade (i.e., with pericardial pressure less then 10 mm Hg) often can be managed without pericardiocentesis. Such patients are managed as hospital in-patients where they are monitored for evidence of progression in the severity of tamponade that first is evident by further rise in the jugular pressure, decreased blood pressure, and some subjective discomfort. If the tamponade was caused by a reversible process such as acute pericarditis, it should vanish after a few days of anti-inflammatory treatment.
Moderate tamponade (i.e., with pericardial pressure about 10 to 15 mm Hg) normally indicates the need for elective drainage of pericardial fluid. Pericardial pressure higher than that means the tamponade is severe and that
drainage is urgent or needs to be performed as an emergency procedure. When the cause of clinically manifest tamponade is not apparent, a search for the etiology is required and includes examination of the pericardial fluid and, less often, pericardial biopsy.
drainage is urgent or needs to be performed as an emergency procedure. When the cause of clinically manifest tamponade is not apparent, a search for the etiology is required and includes examination of the pericardial fluid and, less often, pericardial biopsy.
Tamponade that ensues immediately after pericardial injury (e.g., during a cardiac intervention) requires immediate emergency relief because a small volume of blood can quickly raise pericardial pressure to life-threatening values (Figs. 22-5 and 22-6). Skilled interventional cardiologists can tap these small effusions (31, 32). When this fails, they can access the pericardium through a subxiphoid incision. Catheter techniques, reversal of anticoagulation and fibrinolysis, and life-support measures can stop the bleeding and stabilize the patient. Removal of only some of the blood dramatically improves the patient because it substantially lowers the pericardial pressure (Fig. 22-6).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree