Heart Transplantation
Though major advances in the therapeutic armamentarium have provided improvements in mortality and morbidity for heart failure patients, outcomes are far from optimal. The 1-year survival of patients with New York Heart Association (NYHA) functional class IV heart failure on optimal medical therapy averages 50% to 80%.1 More than 100,000 individuals are estimated to be in end-stage heart failure, while only around 2,000 transplants have been performed annually in the United States for the last many years.2 In contrast to the dismal prognosis of end-stage heart failure patients, the 1-year survival after transplantation averages 85% to 90% at most US centers.2 Hence, heart transplants (and ventricular assist devices) are viable last resort options for heart failure patients to improve mortality and morbidity. Knowledge of the selection criteria and contraindications for heart transplant and timing for consideration of advanced therapies is necessary to provide the best care for heart failure patients. Many heart transplant patients continue to seek care from nontransplant cardiologists, and hence, basic concepts of rejection and immunomodulating pharmacology are necessary in order to suspect rejection and avoid effects of drug interactions. Recent recognition for the need of standardization of care for the cardiac transplant patients has led to development of guidelines by the International Society of Heart and Lung Transplantation (ISHLT).3 The guidelines use categorization of recommendations similar to the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines and are referred to for certain topics thought to be relevant for a general cardiologist.
ORGAN ALLOCATION
There were 16,070 deceased organ donors in the United States during 2007 and 2008, and 28% of the time, a heart was recovered and transplanted.4 The United Network for Organ Sharing (UNOS) has the government contract for the procurement and distribution of cadaveric organs in the United States. UNOS operates through 11 geographic regions of the country with each region further divided into 58 organ procurement organizations (OPOs). The OPO’s main responsibility is to communicate with local hospitals, identify potential donors, and coordinate the transplant process.
THE DONOR
The local OPO is responsible for the initial identification and screening of the potential donor. Once consent for donation is obtained, the primary screening consists of confirming brain death, age, body size, and ABO blood type of the potential donor. The initial evaluation also includes obtaining routine laboratory tests, serologic tests (hepatitis B and C, HIV), identifying the presence of active malignancies, clinical course, and the etiology of death. Cardiac screening includes an electrocardiogram, chest x-ray, transthoracic echocardiogram (if inadequate, transesophageal echocardiogram), and determination of prolonged hypotension or cardiopulmonary resuscitation. A cardiac catheterization may be performed, depending on the age and the presence of coronary artery disease risk factors. Generally, if the potential donor is male and >45 years of age or female and >50 years of age, a cardiac catheterization is recommended.
Once the screening is completed, the donor is entered into the UNOS database and a “rank list” is obtained for each organ. The OPO contacts the recipient transplant centers in order of rank on the list. Candidates are ranked by a variety of factors including ABO blood group compatibility, geographic proximity between donor and transplanting hospitals, priority status of the potential recipient (as detailed below in the recipient section), and length of time spent on the waiting list. In general, the allocation of hearts is first within the local OPO and then outside the OPO in 500-mile concentric rings with the donor hospital in the center of the rings.
The final screen is completed by the harvesting surgeon and involves a review of the locally obtained data as well as a visual inspection of the donor heart. The surgeon looks for contusions, palpates for any obvious atherosclerotic lesions in the epicardial vessels, and reassesses cardiac function.
After brain death has been established and up to the time the organ is procured, the goal of medical management is to maintain hemodynamic stability of the potential donor. Brain death is associated with a high adrenergic state causing fluctuation in blood pressure, arterial vasoconstriction, and end-organ under-perfusion. Therefore, continuous monitoring of arterial pressure, central venous pressure, and urinary output is essential. The targeted systolic blood pressure is >100 mm Hg, central venous pressure between 8 and 12 mm Hg, urinary output >100 mL/h but 300 mL/h, and hematocrit >30%. Close attention must be made to maintaining normal electrolytes, acid–base balance, and oxygenation. The goal is to optimize cardiac output of the donor heart to achieve blood flow that promotes organ function with the least amount of vasoactive drug support. However, inotropic or vasopressor agents (dopamine, dobutamine, and epinephrine) may be needed. Recommendations on donor heart selection have been recently published by the ISHLT, the details of which are beyond the scope of this chapter.3
THE RECIPIENT
Due to disparity between the need and supply of donor hearts, the process of patient selection and allocation must be examined closely. It is crucial that transplant centers allocate organs to patients with the greatest need and the greatest chance to derive maximal benefit.
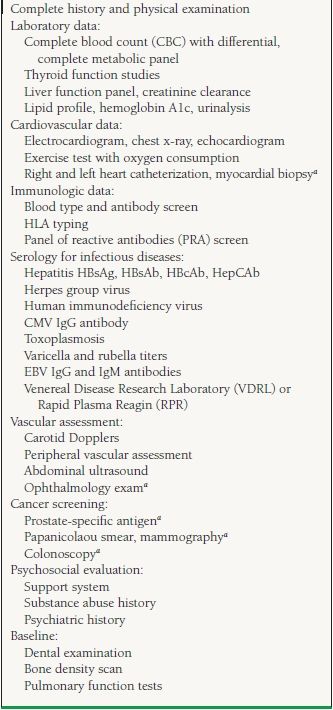
The current indications for cardiac transplantation are listed in Table 17.1. Potential recipients must undergo a thorough evaluation (Table 17.2) in an attempt to identify any condition that could potentially adversely affect the recipient’s survival or quality of life after transplantation. Initial evaluation involves identifying comorbidities that might increase mortality and morbidity independent of heart failure and hence preclude from consideration for transplant.
TABLE
17.1 Selection Criteria for Heart Transplant Listing

TABLE
17.2 Recommended Evaluation Prior to Transplantation
aIf appropriate.
The patient also needs immunologic testing including ABO blood typing, tissue typing for determination of human leukocyte antigens (HLAs), and screening for existing anti-HLA antibodies (Panel Reactive Antigen test). It is imperative that a careful psychosocial evaluation be performed to identify patients with substance abuse, noncompliance, or any behavioral trait that would lead to adverse posttransplant outcomes.
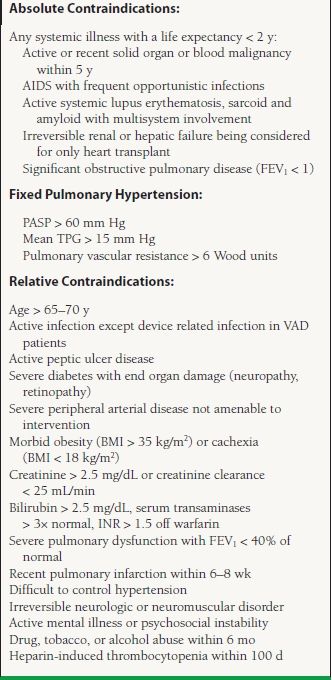
The list of contraindications for transplant listing is evolving constantly. Mancini and Lietz5 published a general list in 2010 and is presented in Table 17.3. There is no consensus for certain relative contraindications and hence the list varies based on individual institution experience and preference.
TABLE
17.3 Exclusion Criteria for Cardiac Transplantation
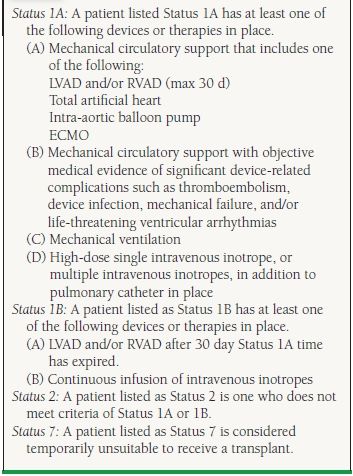
A patient who qualifies for a cardiac transplant gets a priority based on the severity of illness. Hence, the patients on the transplant list are divided into Status 1A (highest priority), defined as patients limited to the intensive care units who are dependent on mechanical circulatory support devices (mechanical assist device, intra-aortic balloon pump, extracorporeal membrane oxygenator) or high-dose intravenous inotropes plus Swan–Ganz catheter. Patients who are mechanically ventilated or have ventricular-assist device-related complications such as a thromboembolism or a device infection and those with a mechanical assist device for a 30 day period are also listed as Status 1A. Status 1B includes patients on continuous intravenous inotropes or patients with ventricular-assist devices once their 30 day 1A time has expired. A patient who does not meet criteria for Status 1A or 1B is listed as Status 2. Status 7 patients are those who are considered temporarily unsuitable to receive a transplant (Table 17.4).
TABLE
17.4 UNOS Status Definitions
Inpatients in need of a transplant are more clinically obvious than ambulatory patients. Determining the appropriate timing for consideration for transplant listing for ambulatory patients can be complicated. A patient should be considered for transplant when the expected survival without transplantation is lower than after transplantation. Variables like ejection fraction, NYHA class, and etiology of heart failure do not predict outcomes in heart failure consistently. In ambulatory patients, one of the best predictors of survival is peak oxygen uptake (VO2) measured by a cardiopulmonary exercise testing, which is an objective measure of functional status. The patient should achieve maximal exercise, represented by respiratory exchange ratio (RER) > 1.05 in order for the VO2 to accurately predict outcomes. In cases where a maximal threshold is not achieved, the carbon dioxide ventilator equivalent ratio (VE/VCO2) >35 can be used as a cutoff to refer for transplant listing. The ISHLT guidelines recommend repeating the test every 6 to 12 months to objectively reassess the need to remain on the transplant list.3 The goal is to list a patient for transplantation after all medical and surgical options have been exhausted, but before the patient becomes debilitated with end-organ damage that may compromise posttransplant survival. Figure 17.1 provides an algorithm for patient selection for transplant listing.
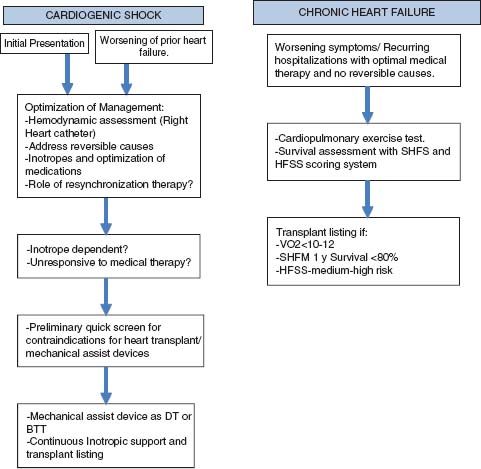
FIGURE 17.1 Steps in considering a patient for transplantation in the setting of acute decomposition (A) and in relatively stable (B) clinical situations. DT, destination therapy; BTT, bridge to transplant.
POSTTRANSPLANT MANAGEMENT ISSUES
Management of heart transplant patients broadly comprises monitoring for and management of the following: (a) allograft rejection, (b) complications and drug interactions of immunosuppressive agents, (c) infections, and (d) malignancies.
Rejection
The transplanted heart is identified as foreign by the recipients’ immune system and is subject to a constant attempt at immune destruction. Cellular identity of self and nonself for an organism is mediated primarily through a group of antigens called major histocompatibility complex (MHC) and expressed on cell surfaces. In humans, this group of proteins is named human leukocyte antigens (as they were first identified on leukocytes).
Rejection can involve both cellular and humoral (antibody-mediated) immune injury to the allograft and is often classified into four major types: hyperacute, acute cellular, antibody-mediated (humoral), and chronic (cardiac allograft vasculopathy [CAV]).
Hyperacute rejection is an antibody-mediated event, which occurs minutes to hours after transplantation and is caused by preexisting recipient antibodies against the donor’s HLA present on the vascular endothelial cells. The histologic hallmark of hyperacute rejection is leaky capillaries, endothelial swelling, microthrombosis, polymononuclear infiltrate, and, subsequently, tissue necrosis. Immunohistochemical studies show deposition of immunoglobulin and complement within the vessel walls. Clinically, there is profound hemodynamic compromise and graft failure. Even with aggressive treatment, hyperacute rejection almost always leads to rapid graft loss. The catastrophic effects of hyperacute rejection can generally be prevented by PRA screening and by donor-recipient HLA and ABO blood- group cross-matching prior to transplantation. It is rarely seen in the current era.
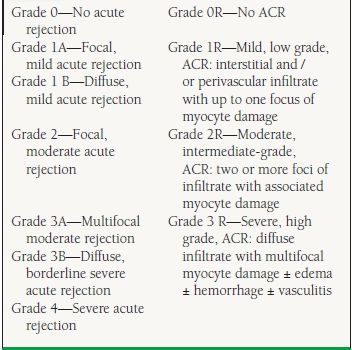
Unlike hyperacute rejection, acute cellular rejection (ACR) is primarily a T-lymphocyte–mediated process, which can occur from the first week after transplantation up to many years out. Twenty to forty percent of transplant patients experience at least one episode of ACR in the first year posttransplantation.6 A histologic grading system was developed in 1990 and updated in 2004 by the ISHLT. This grading classification is based on the amount of inflammatory infiltrate and presence or absence of myocyte necrosis7 (Table 17.5) and helps guide immunosuppressive therapy. The consensus of most transplant centers is that rejection is considered significant when the biopsy is graded at least 2R or if there is any evidence of hemodynamic compromise regardless of grade. Many advanced grades of rejection may be present for prolonged periods (weeks) prior to the development of allograft dysfunction, thus the rationale for “surveillance” biopsies to detect rejection prior to the progression to significant allograft compromise.
TABLE
17.5 The 1990 and Revised 2004 ISHLT Pathologic Grading of ACR
Antibody-mediated rejection, also known as humoral rejection, is initiated by alloantibodies directed against donor HLAs on endothelial cells.8 Antibody-mediated rejection is much less common than ACR. The biopsy reveals arteriolar, venular, and capillary endothelial swelling, nuclear enlargement, and infiltration of macrophages with or without lymphocytes (with B-cell predominance) early in the process. Once complement activation is initiated, there is recruitment of neutrophils, interstitial edema, and intravascular thrombosis with cell injury. Immunoflouresence microscopy shows complement components C3d, C4d, and C1q within the vessel walls. Episodes of antibody-mediated rejection are more severe than ACR and are usually associated with greater hemodynamic compromise, increased incidence of accelerated coronary artery vasculopathy at 1 year, and graft failure, with an overall poorer prognosis. Both AMR and ACR can coexist in 25% of acute rejection episodes.9 Patients at the highest risk for developing humoral rejection include women, patients with high panel reactive antibodies and/or a positive donor-recipient cross-match, cytomegalovirus (CMV) seropositivity, and patients with sensitization to OKT3.10 The 2004 ISHLT pathologic criteria classify AMR into present as AMR1 and absent as AMR 0. The criteria for AMR are (a) evidence of histologic features: myocardial capillary injury with endothelial swelling and intravascular macrophage accumulation with possible interstitial edema and presence of neutrophils and (b) positive immunofluorescence or positive immunoperoxidase staining for AMR (+CD68, C4d). Serologic evidence of donorspecific alloantibodies can be used as a supportive finding but is not required. Though immunoperoxidase staining can be used, immunofluorescence seems to be more sensitive.11 With advancement in laboratory techniques, the concepts, diagnosis, and therapeutics of AMR are evolving.
Monitoring for rejection primarily involves surveillance endomyocardial biopsies (EMBs). Because most rejection episodes occur within the first 3 to 6 months after transplantation, the frequency of biopsies is greater early on. A typical schedule might be weekly for 1 month, every other week for next month, every 3 to 4 weeks for next 1 to 2 months, every 4 to 6 weeks for 1 to 2 months, every 6 to 8 weeks until 1 year after transplant, and then every 3 to 6 months for the next 1 to 3 years while immunosuppression is being altered. Many programs stop routine surveillance biopsies after 3 to 5 years if the patient is stable on maintenance immunosuppression. It is reasonable to consider EMB for unexplained acute graft dysfunction regardless of time posttransplant or if major changes in the immunosuppressive regimen are required. Biopsies for the first 6 to 12 postoperative months and for patients at high risk for rejection beyond 12 months are considered reasonable. The utility of EMBs beyond 5 years is unclear. The current ISHLT guidelines3 do not consider it necessary to perform routine immunostaining techniques screening for AMR, unless there is a suspicion on microscopy. Several centers, however, perform routine immunostaining.
Many studies have evaluated the utility of noninvasive tests such as ECG, signal-averaged ECG, heart rate variability, QT dispersion, echocardiogram, and MRI, but none have shown convincing strength to predict rejection. The only noninvasive technique currently utilized in allograft rejection surveillance is gene expression profiling (GEP). The Allomap test, which utilizes GEP, was approved by the FDA in 2008 for use after 2 months posttransplant. The GEP technique was validated in the Cardiac Allograft Gene Expression Observational (CARGO) study in which peripheral blood mononuclear cells were analyzed for upregulation of certain target genes (which are upregulated during allo- reaction).12 A group of 11 discriminator genes were identified and validated to biopsy evidence of significant rejection (Grade 2R). A scoring system from 0 to 40 is used with the threshold suggesting no rejection varying with the duration from transplant: 3 to 6 months (<20), 6 to 9 months (<30), and >12 months (<34).
The Allomap testing tool can be used for its high negative predictive value in individuals at a low risk of rejection. It is not indicated to be used in those who are <2 months posttransplant, are still on high-dose steroids (≥20 mg prednisone or high dose of IV steroids), have undergone myeloablative therapy in the last 21 days, received blood products or hematopoietic growth factors in the last month, are pregnant, or are <15 years old. The IMAGE trial was undertaken to establish the utility of a clinical strategy of GEP-based rejection surveillance as a noninferior technique compared to the standard of surveillance EMBs.13 In patients >6 months posttransplant, the trial showed that at 1 year, the use of GEP along with clinical and echocardiographic assessment was noninferior (HR 1.04, CI 0.67–1.68) compared with routine EBMs and decreased the number of biopsies per patient. A key question this study raised was the utility of surveillance, irrespective of the type of test, as rejection episodes that were diagnosed prior to clinical graft dysfunction in either arm late after transplant was extremely low.
CAV, often called chronic rejection, remains a major limiting factor to long-term survival following cardiac transplantation. The incidence of CAV after transplant increases with the time from transplant: 8% at 1 year, 20% at 3 years, 30% at 5 years, and >50% at 10 years. It is an aggressive form of coronary artery disease that occurs months to years after transplantation and involves predominantly the entire length of the arterial vasculature with occasional involvement of the veins. Often the small intramyocardial vessels are severely involved. The histologic characteristics show concentric intimal thickening comprising of proliferative smooth muscle cells and extracellular matrix (ECM) with the vascular media and adventitia relatively unaffected. CAV is elicited by endothelial injury with a response from both cellular and humoral immune systems. Immune recognition to endothelial antigens leads to recruitment of inflammatory cells, of which the major effector cells are macrophages. These cells secrete proinflammatory cytokines and chemokines that influence proliferation of smooth muscle cells and deposition of ECM protein, causing luminal occlusion (5). Three stages of evolution of CAV have been suggested: (a) nonspecific endothelial injury (e.g., ischemia, trauma, and infection), (b) allo-response—recruitment of predominantly monocyte—macrophages and T-lymphocytes, and (c) arteriopathy—smooth muscle—like cell proliferation and ECM deposition.
ISHLT GUIDELINES3: REJECTION SURVEILLENCE:
Class IIa:
1. The standard of care for adult heart transplant recipients is to perform periodic endomyocardial biopsy (EMB) during the first 6 to 12 post operative months for surveillance of rejection. (Level of Evidence: C)
2. After the first postoperative year, biopsy surveillance for an extended period of time is recommended in patients at higher risk for late acute rejection, to reduce the risk for rejection with hemodynamic compromise and the risk of death in Africa-American recipients (who are at higher risk of rejection). (Level of Evidence: C)
3. Gene expression profiling can be used to rule out the presence of acute cellular rejection (ACR) of grade 2R or greater in appropriate low-risk patients, between 6 months and 5 years HT. (Level of Evidence: B)
Class IIb:
1. The use of routine EMB later than 5 years after HT is optional, depending on clinical judgment and the risk of for late allograft rejection. (Level of Evidence: C)
Class III:
1. The routine use of ECG parameters for acute allograft rejection monitoring is not recommended. (Level of Evidence: C)
2. The use of echocardiography as an alternative to EMB for rejection monitoring is not recommended (Level of Evidence: C)
3. The routine clinical use of MRI for acute allograft rejection monitoring is not recommended. (Level of Evidence: C)
4. The use of B-type natriuretic peptide (BNP), troponin I or T, C-reactive protein (CRP) levels for acute allograft rejection monitoring is not recommended. (Level of Evidence: C)
5. The use of systemic inflammatory markers for acute heart allograft rejection monitoring is not recommended. (Level of Evidence: C)
Both immune and nonimmune factors contribute to the pathogenesis of CAV The 2010 ISHLT report3 looked at 5,677 transplants performed between July 1997 and June 2001 to determine that the risk of developing CAV within 8 years of transplant was influenced by donor and recipient age, donor diabetes/hypertension, donor body size, and donor/recipient characteristics. Early CAV risk factors include pretransplant coronary artery disease, increase in donor age, donor body mass index (BMI), and a history of donor hypertension, while lesser risks for developing early CAV were seen in female donors and recipients. Late CAV risk factors include donor hypertension, hospitalization during the first year posttransplant for rejection, pretransplant coronary artery disease, HLA-DR mismatch, decreasing recipient age, and increasing donor age.
Unfortunately, the clinical diagnosis of CAV is usually made after the disease is advanced. Many times, the first clinical manifestation of CAV is ventricular arrhythmias, congestive heart failure, or sudden death. The surgical denervation of the heart prevents the pain associated with myocardial ischemia or infarction, particularly in the first 5 to 10 years after transplant. Because of the absence of symptoms, annual angiograms are often performed to detect CAV Angiograms are somewhat insensitive because of the poor visualization of the concentric lesions that affect distal and small vessels before they become apparent in the main epicardial vessels. Coronary angiograms have been shown to underestimate the presence of disease as demonstrated by histopathol- ogy studies and intracoronary ultrasound (IVUS). Studies have shown IVUS to be a more sensitive tool in detecting and following the progression of CAV by identifying maximal intimal thickness (an increase in ≥0.5 mm on serial IVUS examinations is considered significant); however, its increased cost, invasiveness, and lack of universal availability limit its use. An ISHLT consensus document proposes a new prognostically relevant nomenclature of CAV14
Angioplasty of CAV lesions, if discrete, may provide short-term palliation; however, restenosis rates are high and retrospective studies have shown DES to be better than BMS. Coronary artery bypass has limited use because CAV usually involves distal vessels and thus provides poor targets for bypassing. Retransplantation is an option, but the risk is higher than at the first transplant.
The mainstay of therapy for CAV is prevention and modification of coronary artery disease risk factors, including weight loss, lipid reduction, and controlling hypertension, and diabetes. These modifiable risk factors are thought to contribute to endothelial injury and the proliferation of smooth muscle cells and thus progression of CAV. Along with risk-factor modification, certain therapeutic modalities have been shown to be of some benefit in the prevention and progression of CAV. Statins not only lower cholesterol but also downregulate cytokine expression, lower plasma levels of C-reactive protein, and improve endothelial function and hence potentially decrease the onset of CAV. Calcium channel blockers have also been found to stabilize the endothelium and decrease platelet aggregation with a decrease in release of platelet-derived growth factor. Single-center studies have suggested that supplementation with vitamin C and E may retard early progression of transplant-associated arteriosclerosis. Two newer immunosuppressive agents, Sirolimus and Everolimus, have potent antiproliferative and antimigratory actions on vascular smooth muscle cells and have been shown in two multicenter prospective studies to decrease IVUS parameters of coronary vasculopathy. Furthermore, small single-center studies by Mancini et al.15 and Segovia et al.16 (RAPASTAT study) have suggested the benefit of Sirolimus in reducing adverse clinical outcomes and progression of IVUS parameters, respectively, in patients diagnosed with CAV. The clinical use of these proliferation signal inhibitors is limited by poor tolerability related to serositis and infections.
ISHLT GUIDELINES3: SCREENING FOR CAV
Class I:
1. Annual or biannual coronary angiography should be considered to assess the development of cardiac allograft vasculopathy (CAV). Patients free of CAV at 3 to 5 years after transplant, especially those with renal insufficiency may undergo less frequent invasive evaluation. (Level of Evidence: C)
2. Follow-up coronary angiography is recommended at 6 months after a PCI (percutaneous coronary intervention) because of high restenosis rates in transplant recipients. (Level of Evidence: C)
Class II a:
1. Evaluation of Coronary Flow Reserve in conjunction with coronary angiography may be useful for the detection of small vessel coronary artery disease (CAD), which is a manifestation of CAV. (Level of Evidence: C)
2. Treadmill or dobutamine stress echo and myocardial perfusion imaging may be useful for the detection of CAV in heart transplant recipients unable to undergo invasive evaluation. (Level of Evidence: B)



