Heart Failure with Normal Ejection Fraction
Diastolic dysfunction refers to a functional abnormality of myocardial relaxation, distensibility, or filling in the diastolic phase of the cardiac cycle. Heart failure with normal ejection fraction (HFNEF), previously referred to as diastolic heart failure, has increased in prevalence and now accounts for up to 50% of all cases of heart failure. To make the diagnosis of HFNEF, three conditions must be fulfilled: (a) the presence of symptoms or signs of heart failure, (b) the presence of normal or mildly abnormal systolic function, (c) evidence of diastolic left ventricular (LV) dysfunction. HFNEF is increasingly common and can be associated with significant morbidity and mortality, with comparable prognostic outlook among patients with heart failure with reduced ejection fraction (HFREF). Several pathophysiologic definitions of HFNEF have been proposed:
1. Impaired ventricular filling capacity without a compensatory increase in left atrial (LA) pressure
2. Abnormal ventricular filling resulting in inadequate cardiac output with a mean pulmonary venous pressure of <12 mm Hg
3. Resistance to filling of either or both ventricles with an inappropriate shift of the pressure–volume loop.
These definitions all have an abnormal resistance to filling, causing elevated left-sided filling pressures and congestion. Diastolic dysfunction impairs filling of the ventricle by impairing relaxation (early diastole), reducing compliance (early to late diastole), or by external constraint from the pericardium. Numerous pathologic processes and disease states may produce the clinical constellation of diastolic dysfunction. Ultimately, HFNEF is distinguished from HFREF, at the macroscopic level, by concentric LV remodeling, rather than eccentric.
PHYSIOLOGY OF DIASTOLE
Effective diastolic filling depends on the relationship between transmitral LA and LV pressures. Several factors influence this relationship: (a) active myocardial relaxation, (b) intrinsic passive LV compliance, and (c) extrinsic passive properties, including pericardial restraint and ventricular interaction.
PHASES OF DIASTOLE
Diastole is the period from the closure of the aortic valve to the termination of mitral inflow. It is divided into two periods: (a) an isovolumic relaxation period and (b) an auxotonic period that includes rapid filling, diastasis (slow filling), and atrial systole.
Isovolumic Relaxation Phase
The isovolumic relaxation time period occurs from the time of aortic valve closure to mitral valve opening during which there is active relaxation of the contracted myocardium generating a fall in LV pressure without a change in volume. There is active, energy-dependent myocyte relaxation until mid-diastole. Isovolumic relaxation ends when the LV pressure falls below the LA pressure, resulting in mitral valve opening, at which point the rapid filling phase commences.
Rapid Filling Phase
The auxotonic period occurs from mitral valve opening until mitral valve closure. When the LV pressure falls below LA pressure, the mitral valve opens, initiating the rapid filling phase. The elastic recoil and “untwisting” of the ventricle generated by myocardial relaxation creates a suction effect that augments the LA–LV pressure gradient, resulting in rapid filling of the ventricle. Blood acceleration occurs as a result of the development of an LA-to-LV pressure gradient. Blood rapidly enters the left ventricle from the left atrium during the early filling period. In normal hearts, approximately 70% to 80% of LV filling occurs during this phase of diastole. Rapid filling ends as atrial and ventricular pressures equalize.
Diastasis (Slow Filling) Phase
As rapid ventricular filling progresses, LV pressure gradually increases and briefly exceeds LA pressure, resulting in deceleration of mitral inflow and onset of diastasis. Diastasis typically accounts for <5% of filling.
Atrial Filling (Contraction) Phase
The onset of atrial contraction (atrial filling) in late diastole results in a brief increase in the transmitral gradient, forcing blood across the mitral valve and a small amount of regurgitation into the pulmonary veins. In normal hearts, this accounts for 20% to 25% of ventricular end-diastolic volume, with only a small rise in mean pulmonary venous pressure. Diastole ends and systole begins with the onset of ventricular contraction, resulting in a rapid increase in LV pressure that closes the mitral valve.
DETERMINANTS OF DIASTOLIC FUNCTION
Diastolic function depends on four major factors:
1. Active myocardial relaxation. This is mediated by intracellular ATP and calcium. Relaxation results from calcium sequestration into the sarcoplasmic reticulum by the calcium-ATPase pump after contraction. Abnormal relaxation may result from either elevated cytosolic levels of calcium in diastole or inadequate intracellular ATP levels. Factors that may affect isovolumic relaxation include internal loading forces, external loading states, and reduced or inhibited myocardial contractility.
2. Passive pressure–volume relationships (i.e., LV compliance). This is determined by the viscoelastic nature of the myocardium; chamber size, shape, and wall thickness; right and LV pressure–volume interaction; intrathoracic pressure; and pericardial restraint. As LV volume increases during diastole, an increase in LV pressure ensues. The slope of the pressure–volume curve during diastole (dP/dV) represents the chamber stiffness; and the inverse of this relation (dV/dP) is the chamber compliance (Fig. 21.1).
3. Left atrium (including atrial function), pulmonary vein, and mitral valve characteristics. In young, healthy, normal individuals, the atrial contribution is <20% of the total volume, whereas in older normal subjects, this atrial “kick” contributes a greater proportion of total LV filling.
4. Heart rate. As the heart rate increases, the diastolic filling period preferentially decreases with respect to the systolic ejection period.
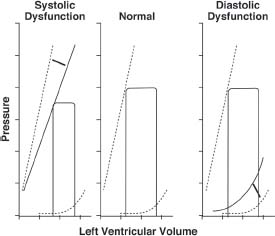
FIGURE 21.1 Schematic representation of ventricular pressure–volume loops. The center panel demonstrates the normal situation. Note the exponential nature of the curve through late diastole. In systolic dysfunction (left), the end-systolic pressure line is displayed downward and is manifest by a decreased ability of the left ventricle to generate high pressures for a given volume. Diastolic dysfunction involves an upward and leftward shift of the exponential curve, a result of elevated filling pressures for a given volume. (Adapted from Katz AM. Influence of altered inotropy and lusitropy on ventricular pressure-volume loops. J Am Coll Cardiol. 1988;11:438–445.)
CLINICAL PRESENTATION
Recent consensus documents have now reported that nearly 50% of patients with heart failure have HFNEF. The clinical presentation of heart failure may include flash pulmonary edema and hypertensive heart disease, advanced ischemic heart disease, or hypertensive hypertrophic cardiomyopathy. Patients who often do not respond to heart failure treatment include patients with aortic stenosis, hypertrophic cardiomyopathy, infiltrative cardiomyopathy, and constrictive pericarditis. Typically, elderly patients with hypertension are at highest risk for developing the clinical syndrome of HFNEF. Signs and symptoms of HFNEF include the following:
1. Dyspnea on exertion and reduced exercise tolerance
2. With disease progression, patients may have dyspnea at rest, paroxysmal nocturnal dyspnea, and orthopnea.
3. Right-sided diastolic dysfunction can cause peripheral edema, bloating, and ascites.
PHYSICAL EXAMINATION
Physical examination cannot effectively separate patients with diastolic heart failure from those with systolic heart failure. Most patients with diastolic heart failure have hypertension or coronary artery disease. On auscultation, an audible S4 (stage I diastolic dysfunction by Doppler echocardiography, indicative of abnormal relaxation) or S3 (stage III diastolic dysfunction by Doppler echocardiography, indicative of reduced compliance) can be heard. There may be pulmonary rales, jugular venous distension, and edema.
LABORATORY EXAMINATION
ECG
The most common abnormality is an LV hypertrophy pattern. LA abnormality may also be seen.
Radiography
There are no specific findings on a chest x-ray. Pulmonary congestion with a normal cardiac silhouette suggests the presence of diastolic dysfunction.
Echocardiography
Echocardiography is the modality of choice to assess for diastolic dysfunction. Findings on an echocardiogram may include the following:
1. Normal LV systolic function and isolated diastolic dysfunction. Patients with abnormal systolic function have secondary diastolic function.
2. LV hypertrophy
3. LA enlargement. LA volume reflects the cumulative effects of filling pressures over time. Accurate measurements of LA volume are obtained using the apical 4-chamber and 2-chamber views, and this assessment is clinically important as there is a significant relationship between LA remodeling and echocardiographic indices of diastolic function. Previous observational studies have shown that LA volume index ≥ 34 m L/m2 is an independent predictor of heart failure, atrial fibrillation, ischemic stroke, and death.
4. Evidence of impaired ventricular filling. This has four stages (Fig. 21.2):
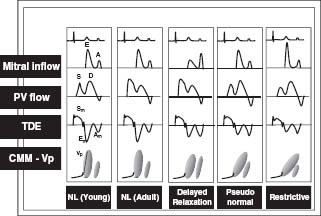
FIGURE 21.2 Stage I or impaired relaxation pattern, stage II or pseudonormal pattern, stage III or restrictive filling pattern, and stage IV or irreversible restrictive pattern. (Adapted from Garcia MJ, Thomas JD, Klein AL. New Doppler echocardiographic applications for the study of diastolic function. J Am Coll Cardiol. 1998;32:865–875.)
 Stage I or impaired relaxation pattern. The time from the peak early (E) wave to the baseline (the deceleration time; DT) is prolonged to >220 milliseconds. The early/atrial (E/A ratio) is <1 and the isovolumic relaxation time is >100 milliseconds. Color M-mode flow propagation slope is <40 cm/s. Tissue Doppler annulus early velocity is <8 cm/s. In addition, the LA volume index should be increased, ≥34 cm/m2.
Stage I or impaired relaxation pattern. The time from the peak early (E) wave to the baseline (the deceleration time; DT) is prolonged to >220 milliseconds. The early/atrial (E/A ratio) is <1 and the isovolumic relaxation time is >100 milliseconds. Color M-mode flow propagation slope is <40 cm/s. Tissue Doppler annulus early velocity is <8 cm/s. In addition, the LA volume index should be increased, ≥34 cm/m2.
 Stage II or pseudonormal pattern. This is associated with a normal appearance of the transmitral inflow pattern with an E/A ratio between 1 and 2, a DT between 150 and 220 milliseconds, and an isovolumic relaxation time between 60 and 100 milliseconds. To distinguish this from normal, the pulmonary venous pattern is analyzed and shows a prolonged and increased atrial reversal time >35 cm/s and the pulmonary venous systolic-to-diastolic flow is normal or <1. Color M-mode reveals a flow propagation slope <40 cm/s. Tissue Doppler annulus early velocity is <8 cm/s.
Stage II or pseudonormal pattern. This is associated with a normal appearance of the transmitral inflow pattern with an E/A ratio between 1 and 2, a DT between 150 and 220 milliseconds, and an isovolumic relaxation time between 60 and 100 milliseconds. To distinguish this from normal, the pulmonary venous pattern is analyzed and shows a prolonged and increased atrial reversal time >35 cm/s and the pulmonary venous systolic-to-diastolic flow is normal or <1. Color M-mode reveals a flow propagation slope <40 cm/s. Tissue Doppler annulus early velocity is <8 cm/s.
 Stage III or restrictive filling pattern. There is reduced LV compliance. Elevated peak E-wave velocity and rapid deceleration are due to increased LV stiffness. The E/A ratio is >2, DT <150 milliseconds, and isovolumic relaxation time is <60 milliseconds. Color M-mode reveals flow propagation slope <40 cm/s, and tissue Doppler annulus early velocity is usually <8 cm/s.
Stage III or restrictive filling pattern. There is reduced LV compliance. Elevated peak E-wave velocity and rapid deceleration are due to increased LV stiffness. The E/A ratio is >2, DT <150 milliseconds, and isovolumic relaxation time is <60 milliseconds. Color M-mode reveals flow propagation slope <40 cm/s, and tissue Doppler annulus early velocity is usually <8 cm/s.
 Stage IV or irreversible restrictive pattern. This stage is similar to the findings of stage III, with no change in the Doppler pattern with preload-reducing maneuvers, and is associated with a substantially increased risk of death.
Stage IV or irreversible restrictive pattern. This stage is similar to the findings of stage III, with no change in the Doppler pattern with preload-reducing maneuvers, and is associated with a substantially increased risk of death.



