Fig. 1
Evaluation of a breathless woman towards the end of pregnancy/early postpartum
Table 1
Pre-conception evaluation and risk assessment in a women with heart failure
Pre-conception evaluation and risk management |
Thorough history of cardiac symptoms and physical examination 12-lead ECG |
Baseline exercise tolerance and functional class |
Baseline echocardiogram |
Assessment of ventricular function (right and left) |
Assessment of pulmonary artery pressure |
Presence and degree of valvular dysfunction |
Assessment of stability of cardiac hemodynamic status over time |
Effective contraception until pregnancy desired |
Adjust medications to prevent adverse fetal events |
Genetics referral for patients with heritable cardiac lesion |
If a patient is hypotensive, or needing inotropes despite optimal medical therapy, she should be transferred to a facility where intra-aortic balloon pump counterpulsation, ventricular assist devices, and transplant consult teams are available. Urgent delivery, irrespective of gestation, may need to be considered in women presenting or remaining in advanced heart failure with haemodynamic instability. As soon as the baby is delivered, and the patient is haemodynamically stable, standard therapy for heart failure can be applied.
Management of Chronic Heart Failure in Pregnancy
For treatment of chronic heart failure the pregnancy status of the patient is important. Patients can be peri- or postpartum. Women who present with heart failure during pregnancy require joint cardiac and obstetric care. Possible adverse effects on the fetus must be considered when prescribing drugs (Fig. 2; Tables 2 and 3).
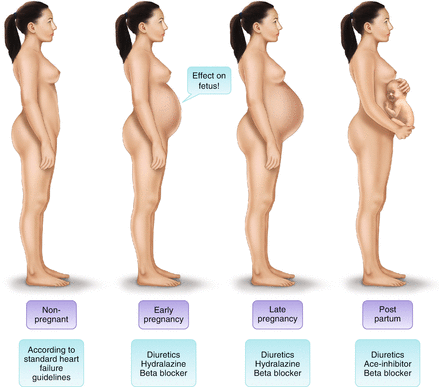

Fig. 2
Treatment of heart failure in women with peripartum cardiomyopathy or other cardiomyopathies according to stage of pregnancy
Table 2
Recommendations for the management of cardiomyopathies and heart failure in pregnancy
Recommendations | Level of evidence |
|---|---|
CLASS I | |
Anticoagulation is recommended in patients with intracardiac thrombus detected by imaging or with evidence of systemic embolism. | A |
Women with HF during pregnancy should be treated according to current guidelines for non-pregnant patients, respecting contraindications for some drugs in pregnancy | B |
Women with DCM should be informed about the risk of deterioration of the condition during gestation and peripartum. | C |
In patients with a past history or family history of sudden death close surveillance with prompt investigation is recommended if symptoms of palpitations or presyncope are reported | C |
Therapeutic anticoagulation with LMWH or vitamin K antagonists according to stage of pregnancy is recommended for patients with atrial fibrillation. | C |
CLASS IIa | |
Delivery should be performed with β-blocker protection in women with HCM. | C |
β-blockers should be considered in all patients with HCM and more than mild LVOTO or maximal wall thickness >15 mm to prevent sudden pulmonary congestion | C |
In HCM, cardioversion should be considered for persistent atrial fibrillation. | C |
CLASS IIb | |
Due to high metabolic demands of lactation and breastfeeding, preventing lactation may be considered in PPCM. | C |
CLASS III | |
Subsequent pregnancy is not recommended if LVEF does not normalize in women with PPCM. | C |
Table 3
Medical management of chronic heart failure in pregnancy according to U.S. Food and Drug Administration (FDA) class
Medical management of chronic heart failure in pregnancy | ||
|---|---|---|
Drug/class | Purpose | Comments |
Diuretics | ||
Furosemide | Generally reserved for treatment of pulmonary edema | Can result in uteroplacental hypoperfusion |
Use of lowest possible dose | FDA class Ca | |
Digoxin | Not considered first-line therapy for heart failure in non-pregnant patients | Generally considered safe |
Useful in treatment of persistent symptoms, despite standard therapy | ||
No improvement in mortality | FDA class C | |
Vasodilators | ||
Hydralazine | Commonly used oral antihypertensive agent in pregnancy | Demonstrated efficacy in hypertension |
Risk of hypotension | ||
Can be substituted for ACE inhibitor during pregnancy | Avoid large or precipitous decreases in blood pressure | |
FDA class C | ||
Aldosterone antagonists | ||
Spironolactone, epleronone | Prolong survival in selected heart failure patients | Not routinely used in pregnancy No data to support safety in pregnancy |
FDA class D | ||
Warfarin | Risk/benefit ratio needs to be discussed with the patient for treatment and prophylactic anticoagulation in severe left ventricular dysfunction | First trimester teratogenesis |
Dosing is complicated in pregnancy | ||
FDA class X (contraindicated) | ||
Heart failure should be treated according to guidelines on acute and chronic heart failure (Gheorghiade et al. 2010). The principal objectives in the management of heart failure are to make patients feel better, reduce hospitalisations (new and recurrent) and to prolong survival. Drugs such as diuretics and digoxin improve symptoms. Beta-blockers, ACE-inhibitors and aldosterone antagonists improve survival. It is now recognized that preventing HF hospitalisation is important for patients and healthcare systems.
Data on the use of medications in pregnancy are limited as pharmaceutical studies often exclude pregnant women due to fear of litigation if fetal effects occur. Evidence is therefore limited and for most medication only a ‘class C’ (Consensus of opinion of experts and/or small studies, retrospective studies or registries) (Regitz-Zagrosek et al. 2011) is available. Levels of evidence on the recommendations for the management of cardiomyopathies and heart failure in pregnancy is summarized in Tables 2 and 3. A number of drugs commonly used in the management of chronic heart failure are not recommended during pregnancy.
Angiotensin Converting Enzyme (ACE) inhibitors, Angiotensin Receptor Blockers (ARBs), and renin inhibitors are contraindicated because of fetotoxicity (Cooper et al. 2006; Bullo et al. 2012).
A recent systemic review by Bullo et al. in Hypertension (Bullo et al. 2012) reported on the use of ACE-inhibitors and ARBs from a total of 72 reports. Thirty-seven articles on 118 well-documented cases described the prenatal exposure to ACE-inhibitors and 35 articles on 68 cases described the use of ARBs. Overall, 52 % of the newborns exposed to ACE-inhibitors and 13 % of the newborns exposed to ARBs did not exhibit any complications (p < 0.0001). Neonatal complications were more frequent following exposure to ARBs and included renal failure, oligohydramnions, death, arterial hypertension, intrauterine growth retardation, respiratory distress syndrome, pulmonary hypoplasia, limb defects and persistent patent ductus arteriosus. Fetal adverse effects by both drugs had relevant neonatal and long-term complications. The outcome was poorer following exposure to ARBs versus ACE-inhibitors. The authors rightly concluded that relevant complications are regularly described, indicating that awareness of the deleterious effects of prenatal exposure to drugs inhibiting the renin-angiotensin system should be improved.
For symptomatic relief, and to reduce afterload, hydralazine and nitrates can be used instead of ACE inhibitors/ARBs for afterload reduction. Diuretics should only be used if pulmonary congestion is present since they may decrease blood flow to the placenta. Furosemide and hydrochlorothiazide are most frequently used.
For symptomatic relief, managing tachycardia and improving long-term outcome, beta-blockers can be considered, carefully weighing up the benefit for the mother versus the possible impaired outcome for the fetus and newborn baby. Data on beta-blocker use in pregnancy are limited and conflicting. However, a recently published survey from a Danish birth cohort (Meidahl Petersen et al. 2012), comprising all births in Denmark between 1995 and 2008, explored the effect of beta-blockers on pregnancy outcomes. The authors identified 2,459 pregnancies exposed to beta-blockers. Interestingly, Danish pharmacies are required by law to register all redeemed prescriptions and, therefore, this study included data on exposure to beta-blockers based on information on prescriptions paid for and not only prescribed by the physician. The authors defined being born small for gestational age as having a birth weight below the 10th percentile for the corresponding gestational week. Preterm birth was defined as born before the 37th gestational week. Beta-blocker exposure during pregnancy was found to be associated with increased risk of small for gestational age (SGA) fetuses (adjusted OR 1.97, 95 % CI 1.75–2.23), preterm birth (adjusted OR 2.26, 95 % ci 2.03–2.52) and an increased perinatal mortality (adjusted OR 1.89, 95 % CI 1.25–2.84). The authors found similar risks irrespective of type of beta-blocker used. However, the study has a major limitation in the way that the authors could not adjust for the treatment indication and severity of the maternal disease, nor were they able to rule out confounding factors. Maternal disease could also possibly explain the findings.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


