Fig. 1.1
ACCF/AHA stages of HF according to risk and symptoms (Reproduced with permission from JACC [5])
NYHA classes focus on exercise capacity and symptoms of HF (see Chap. 9):
NYHA class I patients with no limitation of physical activity and ordinary physical activity does not cause symptoms of HF
NYHA class II slight limitation of physical activity, comfortable at rest, but ordinary physical activity results in symptoms of HF
NYHA class III marked limitation of physical activity, with patient being comfortable at rest, but less than ordinary activity causes symptoms of HF
NYHA class IV patients who are unable to carry on any physical activity without symptoms of HF or symptoms of HF at rest
1.4 Evaluation of a HF Patient
Evaluation of a HF patient includes a thorough history and physical examination, ascertainment of symptoms, functional capacity, and volume status including ascertainment of dyspnea on exertion, orthopnea, paroxysmal nocturnal dyspnea, fatigue, and lower extremity edema. Etiology, comorbidities, and contributing factors for HF should be addressed including presence of diabetes; hypertension; smoking; prior cardiac disease; family history of cardiac disease, HF, or cardiomyopathy; history of heart murmur, congenital heart disease, and rheumatic fever; sleep disturbances; thyroid disease history; exposure to infectious etiology; exposure to cardiotoxins; and past or current use of alcohol and illicit drugs.
Pertinent physical examination includes heart rate and rhythm; blood pressure; measurements of weight, height, and body mass index; overall volume status; jugular venous distension; carotid upstroke and presence/absence of bruits; lung examination for rales or effusions; cardiac examination for systolic or diastolic murmurs; displaced PMI (point of maximum impulse); presence of left ventricular heave; intensity of the second heart sound (S2); presence of third or fourth heart sound (S3 or S4); liver size; presence of ascites; presence of renal bruits; presence of abdominal aortic aneurysm; peripheral edema; peripheral pulses; checking whether the extremities are cold and clammy.
Initial laboratory evaluation of patients presenting with HF should include complete blood count, urinalysis, serum electrolytes (including calcium and magnesium), blood urea nitrogen, serum creatinine, glucose, fasting lipid profile, liver function tests, and thyroid-stimulating hormone [5]. Screening for hemochromatosis, human immunodeficiency virus (HIV), pheochromocytoma, amyloidosis, or rheumatologic diseases reasonable in selected patients, particularly if there is clinical suspicion for testing [5] (Table 1.1).
Table 1.1
Initial diagnostic work-up of a HF patient
Detailed history | Detailed history for causes of HF, review of comorbidities, medications, social history, drug or substance use, cardiotoxin or infectious exposure, pregnancy. In patients with idiopathic DCM, a 3-generational family history should be obtained to aid in establishing the diagnosis of familial DCM |
Initial diagnostic work-up | Complete blood count with differential |
Metabolic panel: serum electrolytes including glucose, calcium, magnesium, BUN, creatinine, HbA1c | |
Urinalysis | |
Thyroid function tests | |
Liver function tests | |
Chest radiography | |
Echocardiography | |
12-Lead electrocardiography | |
Measurement of BNP or NT-proBNP to support clinical judgment for the diagnosis of acutely decompensated HF, especially in the setting of uncertainty for the diagnosis | |
Screening for or HIV, hemochromatosis, rheumatologic diseases, amyloidosis, or pheochromocytoma in patients at risk or with clinical suspicion | |
Other testing that may be considered according to initial clinical assessment and further indications | Cardiac MRI to assess for myocardial infiltrative processes |
Cardiac catheterization for coronary or hemodynamic assessment | |
Invasive hemodynamic monitoring with a pulmonary artery catheter to guide therapy in patients who have respiratory distress or clinical evidence of impaired perfusion in whom the adequacy or excess of intracardiac filling pressures cannot be determined from clinical assessment | |
Ischemia and viability assessment in patients with ischemic heart disease | |
Endomyocardial biopsy in patients presenting with HF when a specific diagnosis is suspected that would influence therapy | |
Cardiopulmonary exercise testing to assess for functional capacity and or consideration for cardiac transplantation |
Initial cardiac evaluation includes a baseline electrocardiogram (ECG); chest X-ray; and a 2-dimensional echocardiogram with Doppler should be performed to assess ventricular function, size, wall thickness, wall motion, and valve function [5] (Table 1.1). Cardiac magnetic resonance imaging is reasonable when assessing myocardial infiltrative processes or scar burden. Biomarkers, especially natriuretic peptides, are useful to support clinical decision making regarding the diagnosis of HF and establish prognosis both in chronic ambulatory or acutely decompensated/hospitalized HF patients [5]. Natriuretic peptide-guided HF therapy can be useful to achieve optimal dosing of guideline-directed medical therapy (GDMT) in select clinically euvolemic patients followed in a well-structured outpatient HF disease management program, while the usefulness of serial measurement of BNP or NT-proBNP to reduce hospitalization or mortality in patients with HF or the usefulness of BNP- or NT-proBNP-guided therapy for acutely decompensated HF is not well established. Cardiac troponins and other evolving biomarkers can be helpful with prognosis and risk stratification of HF patients (see Chaps. 10, 11, and 12).
1.5 Current Management Strategies in HF
1.5.1 Guideline-Directed Medical Therapy (GDMT)
The 2013 ACCF/AHA guideline for the management of HF provides a comprehensive guide to evaluation and management of HF patients [5]. Guideline-directed medical therapy (GDMT), which represents the optimal medical therapy recommended with a class I indication in patients with systolic HF, includes ACE inhibitors (ACE-I), angiotensin receptor blockers (ARBs) when ACE-I intolerant, β-blockers (specifically, bisoprolol, carvedilol, and extended-release metoprolol), and, in select patients, aldosterone receptor antagonists, hydralazine-nitrates, and diuretics as the mainstay of pharmacological therapy for HFrEF (Fig. 1.2) (see Chaps. 8, 36, 38, and 40). It should be noted that indications for aldosterone antagonists for symptomatic HFrEF patients include mild to moderate HF (NYHA class II) patients with a history of a prior cardiovascular hospitalization or elevated plasma natriuretic peptide levels. Additionally existing indications include NYHA class III and IV HF patients with severe HF [5] but with safeguards of creatinine ≤2.5 mg/dL in men or ≤2.0 mg/dL in women and potassium ≤5.0 mEq/L along with the necessity for careful monitoring of potassium, renal function, and diuretic dosing at initiation follow-up in patients treated with aldosterone antagonists. Routine combined use of an ACE inhibitor, ARB, and aldosterone antagonist is considered potentially harmful and is not recommended [5]. The combination of hydralazine and isosorbide dinitrate is recommended in African-American patients with NYHA class III–IV HFrEF and is considered potentially useful in patients who are ACE inhibitor or ARB intolerant. Digoxin similarly is potentially beneficial in patients with HFrEF to decrease hospitalizations for HF (remains a class IIa recommendation) [5].
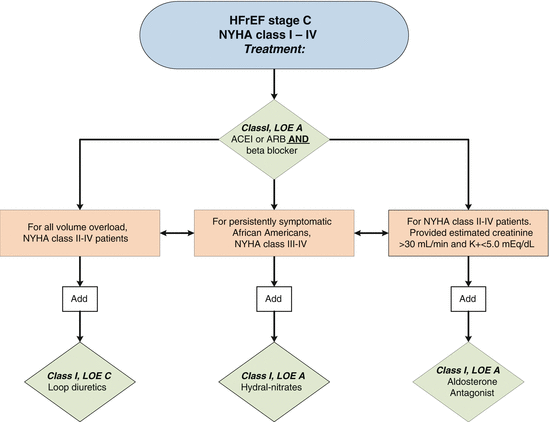

Fig. 1.2
Evidence-based, guideline-directed medical therapy in symptomatic stage C (NYHA class I–IV) HF patients with reduced ejection fraction (HFrEF) (Reproduced with permission from JACC [5])
1.5.2 Device Therapy
Implantable cardioverter defibrillator (ICD) is recommended for primary prevention of sudden cardiac death in selected patients with LVEF ≤35 % and NYHA class II or III symptoms, who have reasonable expectation of meaningful survival for more than 1 year [5] (Chap. 8).
Cardiac resynchronization therapy (CRT) is recommended in patients who have LVEF ≤35 %, sinus rhythm, left bundle branch block (LBBB) with a QRS duration of ≥150 ms, and NYHA class II, III, or ambulatory IV symptoms on GDMT. CRT can be useful in patients with LBBB but QRS duration of only 120–149 ms or those with non-LBBB pattern and QRS ≥150 ms. Of note, for patients with non-LBBB and QRS 120–149 ms, the CRT indication is not expanded beyond patients with NYHA class III/ambulatory class IV; and in patients with non-LBBB and QRS <150 ms and with NYHA class I or II symptoms, CRT or ICD is not indicated in patients in whom cardiac or noncardiac comorbidity and/or frailty limit survival with good functional capacity to less than 1 year [5].
Mechanical circulatory support (MCS) can be considered in select advanced HF patients in whom definitive management such as cardiac transplantation is planned (i.e., as a “bridge to transplant”); or cardiac recovery is anticipated (i.e., as a “bridge to recovery”), or as “destination therapy.” Nondurable MCS, including the use of percutaneous and extracorporeal ventricular assist devices, is considered reasonable as a “bridge to recovery” or a “bridge to decision” for carefully selected patients with acute, profound hemodynamic compromise. These considerations are in line with the current patient care spectrum, reflecting a higher and broader use of these devices in different clinical scenarios [5].
1.5.3 Acute Decompensated HF
In acute decompensated hospitalized HF patients, intravenous loop diuretics such as furosemide, torsemide, and bumetanide remain as first-line therapy. When diuresis is inadequate, it to be reasonable to intensify the diuretic regimen using either higher doses of intravenous loop diuretics or adding a second (e.g., thiazide) diuretic. In the absence of hypotension, intravenous vasodilators such as nitroglycerin, nitroprusside, or nesiritide may be considered as an adjunct to diuretic therapy for relief of dyspnea in patients admitted with acutely decompensated HF [5].
1.6 Emerging Therapies in HF
Some of the emerging therapies in HF are reviewed below, and strategies such as gene therapy and microRNA therapeutic are also discussed at length in Chap. 14 and 15.
1.6.1 Cardiac Inotropes
Currently used inotropic agents have failed to show benefit beyond short-term hemodynamic improvements in patients with HF [6]. These include cardiac glycosides, β-adrenoceptor agonists, phosphodiesterase (PDE) inhibitors, and calcium sensitizers. Heightened energy utilization and the coupling of contractility, chronotropy, and calcium represent significant limitations to their use. Not only do they induce maladaptive remodeling by increasing metabolic demands on the heart, they are also pro-arrhythmic. Increased arrhythmias associated with their use increase mortality and morbidity in patients with decompensated HF. Two novel therapies attempting to dissociate inotropy and arrhythmogenicity are cardiac myosin activators such as omecamtiv mecarbil and istaroxime.
Cardiac myosin activators (CMA) are drugs that directly target the force-generating cardiac enzyme and myocardial myosin ATPase, accelerating its activity in order to enhance contractility. They increase cardiac myosin ATPase, enhancing the release of inorganic phosphate, which strengthens binding between myosin and actin, leading to shortening of the cardiac sarcomere. CMAs increase the efficiency with which ATP is utilized without increasing ATP consumption by increasing the number and duration of actin-myosin crossbridges for each ATP molecule consumed. This prolongs systole but not the rate at which force is developed. This is unlike conventional inotropic agents that generally increase ATP consumption and increase the velocity of contraction and rate of force generation but may shorten the duration of systole. Importantly, CMAs do not possess phosphodiesterase activity, do not increase diastolic calcium concentrations, and can increase cardiac performance in patients receiving beta-blockers. In the phase II Acute Treatment with Omecamtiv Mecarbil to Increase Contractility in Acute HF (ATOMIC-AHF) study, omecamtiv mecarbil did not achieve its primary efficacy endpoint in reducing dyspnea in patients with acute HF. However, a cohort which received the highest dose of the drug showed greater dyspnea relief compared with placebo. Chronic Oral Study of Myosin Activation to Increase Contractility in HF (COSMIC-HF) is a double-blind, randomized, placebo-controlled, multicenter, dose escalation study designed to assess the pharmacokinetics and tolerability of three oral modified-release formulations of omecamtiv mecarbil in patients with chronic HF and left ventricular systolic dysfunction. Calcium dynamics play a prominent role in cardiac function, and its abnormalities contribute to several cardiac diseases including HF, which is discussed at length in Chap. 4.
Istaroxime, an inhibitor of Na+/K+-ATPase and an activator of sarcoplasmic reticulum calcium pump (SERCA), is a new luso-inotropic compound that stimulates cardiac contractility and relaxation in healthy and failing hearts in animal models and in patients with acute HF syndrome. The HORIZON-HF trial evaluated the hemodynamic, echocardiographic, and neurohormonal effects of intravenous istaroxime in 120 patients hospitalized with HF and reduced ejection fraction. In this randomized, double-blind, placebo-controlled, dose-escalating study, three doses of istaroxime or a placebo were given as intravenous infusions over 6 h to patients with a history of HF and a pulmonary capillary wedge pressure (PCWP) over 20 mmHg [7]. A reduction in PCWP was the primary endpoint, which was attained in all three dose groups during the entire observation period of 6 h. There was an increase in systolic blood pressure and a transient increase in cardiac index with the highest dose and a decrease in heart rate and diastolic and systolic volume, without a change in ejection fraction. Echocardiographic indicators of diastolic function also showed improvement. The limitation of this study is related to the fact that patients included presented with milder forms of acute HF, not requiring inotropic interventions.
Research involving gene therapy approaches to increase sarcoplasmic reticulum calcium pump activity and is also ongoing (Chap. 15).
1.6.2 Neurohormonal Modulation
The renin-angiotensin aldosterone system (RAAS) represents a long established therapeutic target in cardiovascular disease, and multiple inhibitors of the pathway have been shown to improve outcomes in chronic HF. However, the inhibition of downstream pathway activity can produce a compensatory rise in plasma renin activity that can competitively overcome RAAS blockade. Hence, aliskiren, a direct renin inhibitor, was studied in the Aliskiren Trial on Acute HF Outcomes (ASTRONAUT) [8]. This international, double-blind study enrolled stable patients hospitalized for HF and followed them after discharge. Patients were randomized to receive either aliskiren, starting at 150 mg and increasing to 300 mg, or placebo, in addition to other standard HF therapies. After 6 months, patients in both groups had a similar likelihood of cardiovascular death or rehospitalization for HF. Despite a significant and sustained reduction in natriuretic peptide level, aliskiren did not reduce mortality or rehospitalization rates. It is possible that a beneficial effect on HF progression, as suggested by this long-term improvement in natriuretic peptide level, was offset by potential negative drug-associated effects, such as hyperkalemia, hypotension, and worsening renal function, particularly in patients with diabetes mellitus (Chap. 36).
More recently, the results of the PARADIGM-HF trial were presented at the European Society of Cardiology meeting where the angiotensin receptor neprilysin inhibitor LCZ696 was superior to enalapril in reducing the risk of death and of hospitalization for HF [9]. Neprilysin, a neutral endopeptidase, degrades several endogenous vasoactive peptides, including natriuretic peptides, bradykinin, and adrenomedullin. Inhibition of neprilysin increases the levels of these substances, countering the neurohormonal overactivation that contributes to vasoconstriction, sodium retention, and maladaptive remodeling. Combined inhibition of the renin-angiotensin system and neprilysin had effects that were superior to those of either approach alone in experimental studies, but in clinical trials, the combined inhibition of ACE and neprilysin was associated with serious angioedema. LCZ696, which consists of the neprilysin inhibitor sacubitril (AHU377) and the ARB valsartan, was designed to minimize the risk of serious angioedema.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


