11 Heart Failure Caused by Congenital Heart Disease
Basic Principles
Key Points
• Heart failure occurs frequently in children with congenital heart disease (CHD) and is often an indication for intervention.
• In contrast to adult heart failure, which is often caused by decreased coronary perfusion, children with CHD typically develop heart failure as a result of the underlying cardiac defect or in association with congenital heart surgery.
• Commonly used measures of ventricular performance (Table 11-1) are limited because they are preload and/or afterload dependent. However, in serial assessment of function, these methods are useful clinically.
• Newer echocardiographic modalities to assess ventricular performance (such as strain and strain-rate imaging) are challenging in the CHD population because of the abnormal anatomy.
TABLE 11-1 ECHOCARDIOGRAPHIC INDICES OF VENTRICULAR PERFORMANCE
| Shortening fraction (SF) |  |
| Ejection fraction (EF) |  |
| Heart rate corrected mean velocity of circumferential fiber shortening (Vcfc) |  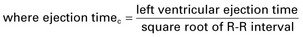 |
| End-systolic wall stress (ESWS) |  |
| Myocardial performance index (MPI) |  |
The Causes of Heart Failure in CHD
Left-to-Right Shunts
• Left-to-right shunt lesions comprise the most common congenital cardiac anomalies in children, excluding bicuspid aortic valve (Box 11-1), and are the most common cause of high-output heart failure.
• Newborn infants with significant left-to-right shunts are typically asymptomatic at birth as a result of high pulmonary vascular resistance. If a left-to-right shunt is large (i.e., a large ventricular septal defect), symptoms usually develop by 4 to 8 weeks of age when the pulmonary vascular resistance normalizes.
• Over this time period, pulmonary blood flow increases, resulting in symptoms that include tachypnea, tachycardia, failure to gain weight, and diaphoresis with feedings. Feeding is a form of exercise for the newborn, thus nursing or bottle-feeding in a child with a large left-to-right shunt will often result in a catecholamine surge.
• As a rule, surgical repair of left-to-right shunts alleviates the heart failure and growth resumes.
Key Points
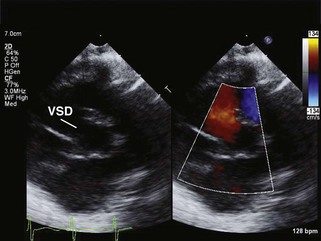
• Echocardiography of left-to-right shunts will identify the lesion in multiple views (Figure 11-1).
• If the shunt is large, the pressure in the right and left ventricles will be equal and Doppler color interrogation will demonstrate laminar flow (see Figure 11-1).
• Other indicators of a chronic, large shunt will include chamber dilatation, generally best seen in the subxiphoid and apical 4-chamber views (see Video 11-1 on the Expert Consult website). In ventricular septal defect, common atrioventricular canal defect, truncus arteriosus, and patent ductus arteriosus, the left atrium and left ventricle dilate because of left ventricular volume overload.
• Right ventricular pressure will be systemic or near-systemic with a large left-to-right shunt and can be measured from the tricuspid regurgitation jet or from the velocity across the ventricular septal defect.
• Ventricular systolic performance is typically hyperdynamic (as measured by shortening fraction or ejection fraction) to compensate for the high cardiac output. If left unrepaired for a long time, ventricular dysfunction may occur.
• In contrast, atrial septal defect and partial anomalous pulmonary venous return result in right atrial and right ventricular dilatation. Of note, these two lesions do not typically cause early heart failure.
Left Heart Obstructive Lesions
• Left ventricular systolic dysfunction is seen with higher frequency in certain congenital heart lesions, such as left heart obstructive lesions (Box 11-2).
• The pressure load associated with these obstructive lesions results in ventricular hypertrophy, elevated end-diastolic pressure and eventual systolic and diastolic dysfunction.
• Critical left heart obstruction is a newborn diagnosis, defined as obstruction that is so severe that the infant is dependent on flow from the ductus arteriosus to augment cardiac output. Neonates with critical left heart obstruction require urgent surgical or catheter-directed intervention.
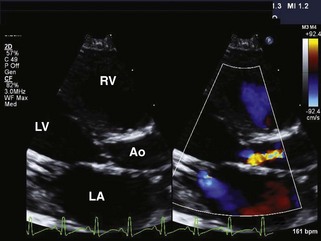
• Left ventricular failure is a component of the diagnosis because of significant pressure overload from the diminutive aortic valve orifice (Figure 11-2; also see Video 11-2 on the Expert Consult website). In addition, some infants with critical aortic stenosis have marked mitral regurgitation, which adds a volume load to the already pressure-loaded left ventricle (see Video 11-2 on the Expert Consult website).
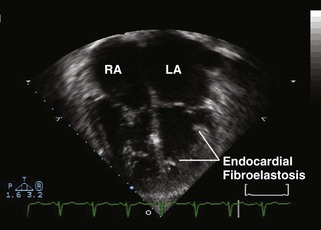
• Critical aortic stenosis is often associated with endocardial fibroelastosis. Endocardial fibroelastosis is a poorly understood phenomenon whereby the left ventricular endocardium becomes fibrotic, likely as a result of long-standing subendocardial ischemia (Figure 11-3; also see Video 11-3 on the Expert Consult website). Children with endocardial fibroelastosis have poor ventricular systolic shortening and high end-diastolic and left atrial pressure, even after the aortic stenosis is relieved.
• After intervention, these infants may remain quite ill because of residual outflow obstruction, poor left ventricular function or the development of new-onset aortic regurgitation as a complication of the intervention.
• Intervention for aortic stenosis in later infancy or childhood tends to have better outcome because the left ventricle has responded over time to the pressure load by developing hypertrophy.
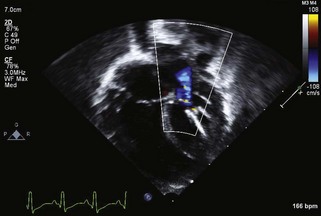
Figure 11-3 Apical 4-chamber view in the same patient as Figure 11-2 with critical aortic stenosis demonstrates a dilated but hypoplastic left ventricle (non–apex forming), with echo-bright papillary muscles consistent with endocardial fibroelastosis. Color Doppler demonstrates mitral regurgitation.
Key Points
• Endocardial fibroelastosis can often be visualized in multiple views, but the sonographer must be cautioned not to mistake increased gain for this disorder.
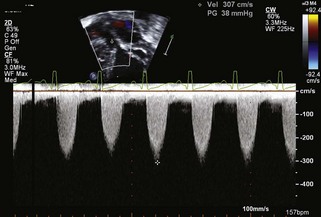
• In critical aortic stenosis, it must be recognized that the peak instantaneous velocity across the stenotic aortic valve may not be particularly high if there is severe left ventricular dysfunction, because the ventricle cannot generate high pressure (Figure 11-4).
• Additional echocardiographic evidence of low cardiac output is a shortened ejection time using pulsed wave Doppler.
• In critical left heart disease, the ductus arteriosus flow pattern will be right to left in systole to augment cardiac output from the right ventricle.
• Adolescents and young adults who had critical aortic stenosis in association with endocardial fibroelastosis as infants may continue to show evidence of significant cardiac disease, which may be manifested as restrictive cardiomyopathy (Figure 11-5; also see Video 11-4 on the Expert Consult website).
The Systemic Right Ventricle
• Certain forms of CHD, both palliated and unpalliated, result in the right ventricle supporting the systemic circulation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree