Classification
EF
Description
I. Heart failure with reduced ejection fraction (HFrEF)
≤40
Alo referred to as systolic HF
Efficacious therapies well demonstrated to date.
II. Heart failure with preserved ejection fraction (HFpEF)
≥50
Also referred to as diastolic HF.
Diagnosis of exclusion
Efficacious therapies have not yet been identified
(a) HFpEF, borderline
41–49
Intermediate group.
Treatment and outcomes similar to HFpEF
(b) HFpEF, improved
>40
Subset of HFpEF patients where EF improved or recovered.
May be distinct group
Needs to be characterized better
In 2005, the American College of Cardiology/American Heart Association (ACC/AHA) introduced a new staging system for heart failure [44]. This staging system remains unchanged in the recently released 2013 ACC/AHA guidelines (Table 8.2) [10]. The purpose was to highlight that heart failure is a progressive syndrome with a spectrum ranging from asymptomatic patients with risk factors to end-stage disease. The first two stages (A and B) comprise asymptomatic patients and may account for over 50 % of the population, based on this new classification system [10]. Stage A includes only patients with risk factors for the development of heart failure such as diabetes, hypertension, obesity and coronary artery disease. Emphasis has been placed on early recognition of risk factors and targeted, aggressive treatment in order to prevent progression to the more advanced disease stages. Stage B includes patients with evidence of structural heart disease such as left ventricular systolic dysfunction or left ventricular hypertrophy but without symptoms related to heart failure. Early appropriate therapy such as the use of beta-blockers and ACE inhibitors in patients with asymptomatic low ejection fraction reduces mortality and delays the development of symptomatic disease [10]. Patients with evidence of structural heart disease and current or prior symptoms of heart failure are classified as Stage C. The vast majority of clinical trials have targeted this stage, including pharmacological agents and device therapies. Finally, Stage D classification includes patients who have advanced end stage heart failure and may require specialized interventions, including inotropic therapy, heart transplantation and left ventricular assist device placement. Current estimates suggest that there are approximately 100,000–250,000 patients in the United States with Stage D heart failure who may be eligible for these therapies [10]. The New York Heart Association (NYHA) has created a functional classification system for describing the severity of symptoms including NYHA Class I–IV (Table 8.3).
Table 8.2
ACC/AHA Guidelines Staging System
Stage | Description |
|---|---|
A | Patients at high risk for developing heart failure who have no structural heart disease or symptoms of heart failure. |
B | Patients who have not yet developed symptoms of heart failure but have structural heart disease. |
C | Patients with a history of symptoms of heart failure with associated underlying structural heart disease. |
D | Patients with end stage heart failure requiring advanced treatments. |
Table 8.3
New York Heart Association Classification
Class | Description |
|---|---|
I | Patients with cardiac disease but without symptomsa or limitations in physical activity. |
II | Patients with cardiac disease resulting in mild symptoms and slight limitations in ordinary activity. |
III | Patients with cardiac disease resulting in symptoms with less than ordinary activity. |
IV | Patients with cardiac disease resulting in the inability to carry out any physical activity without symptoms. There are symptoms at rest. |
When evaluating women for their likelihood of developing heart failure it is important to know the risk factors for heart failure as they relate specifically to gender. Coronary artery disease (CAD), the most prevalent risk factor for the development of heart failure is more common in males than in females [11]. However, females suffer from a higher prevalence of both hypertension and diabetes compared to males and their presence appears to confer a higher risk for developing heart failure [12, 13] (Fig. 8.1).
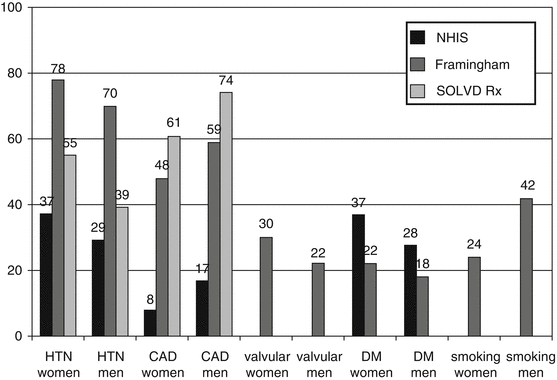

Fig. 8.1
Prevalence of risk factors in the HF population in the NHIS, Framingham and SOLVD studies (Reproduced with permission from Lund and Mancini [88])
The presence of obesity also increases the risk for heart failure in females more so than in males [13]. The Framingham study suggests that overall prognosis in women is significantly better than in men after the onset of heart failure [14]. This may be due to the fact that women have less ischemic cardiomyopathy, and survival might be related to sex differences in etiology. However, due to the longer lifespan of women compared to men as well as the increased incidence of heart failure in older females compared to males, the overall total number of heart failure-related deaths may be higher in women [14].
It is important to recognize that heart failure is a clinical syndrome that largely relies on history and physical exam for an accurate diagnosis. The symptoms of both HFREF and HFPEF are similar between men and women and in general, patients with heart failure will present in one of three ways. These include (1) decreased exercise tolerance, typically with complaints of dyspnea on exertion or fatigue (2) fluid retention and weight gain manifesting as leg and/or abdominal swelling and (3) asymptomatic or with symptoms of another cardiac or non-cardiac disorder such as an arrhythmia, pulmonary or systemic thromboembolic event [10]. Physical exam findings of increased jugular venous pressure and the presence of the hepato-jugular reflex correspond to elevated pulmonary capillary wedge pressures and are highly sensitive findings in heart failure [15]. During the cardiovascular exam the presence of an S3 represents a poor prognostic indicator [15]. Patients may also have an S4, which may indicate diastolic dysfunction. The presence of any of a variety of murmurs may indicate possible valvular disease. Lung exam findings of rales and dullness at the lung bases are consistent with pulmonary edema and/or pleural effusion. Of note, the presence of these pulmonary physical exam findings lack diagnostic sensitivity in chronic heart failure [15]. Additional findings consistent with more advanced, low output heart failure include cold and pale extremities, altered sensorium and decreased urine output.
Laboratory investigation should include a basic metabolic panel to assess for potential abnormalities in serum creatinine, blood urea nitrogen and sodium, all of which serve as negative prognostic markers in heart failure [16, 17]. Anemia noted on a complete blood count (CBC) is also a well-known negative prognostic marker [18]. The clinical use of natriuretic peptides (BNP and proBNP) in heart failure are potentially numerous and have both diagnostic and prognostic utility [19]. The recent 2013 AHA/ACC heart failure guidelines identify the use of natriuretic peptides as useful elements to achieved optimal dosing of guided directed medical therapy in selected patients, while acknowledging that their usefulness of serial measurement to reduce outcomes is not well established [10]. Thus their use is strictly as an adjunct to that of the history and physical exam. In females, natriuretic peptide levels are typically elevated when compared to men [20]. In addition, when compared with HFrEF, natriuretic peptide levels are lower in HFpEF, a population in which females outnumber males [21]. A chest x-ray can provide quick visualization of heart and lung to determine if there is cardiomegaly, pulmonary edema, pulmonary vascular congestion, and pleural effusions. ECG which can show evidence of myocardial ischemia, injury, or infarction, atrial enlargement, left ventricular hypertrophy (LVH), or low voltage. Echocardiography is the gold standard imaging study for the evaluation of heart failure and can be used to determine the presence of systolic and diastolic function as well as hypertrophy, valvular and pericardial disease. As previously noted, females tend to present with concentric hypertrophy more than males, who have a higher prevalence of eccentric hypertrophy [7]. Concentric LVH and left atrial enlargement are characteristic structural changes noted on echo study in HFPEF and among the very elderly, over 80 % of women were found to display LVH [22].
Therapy of HF in Women
Management of HF with Reduced Ejection Fraction (HFrEF)
Current evidence is inconclusive as to whether or not clinical differences exist between men and women with HFREF using standard heart failure treatment. This is due to the overall low percentage of females enrolled in these studies and the small amount of gender specific data recovered from these studies. Recommendations for the treatment of women with heart failure have been made by the Heart Failure Society of America whose conclusions derived from this limited database are simply that the management of HFREF should remain similar for both women and men. The following section will therefore summarize what we currently know in regards to the management of HFREF and HFPEF and how this may be approached in the female population.
Angiotensin converting enzyme inhibitors (ACE-I) are an established first line therapy for chronic HFREF and the mechanism of their benefit is likely secondary to decreasing the maladaptive neurohormonal response by the renin angiotensin aldosterone system in the syndrome of heart failure. ACE-I have been shown to decrease short-term mortality by 40 % in patients with advanced heart failure [23] and by 16 % in patients with less advanced disease [24]. ACE-I also decrease mortality in patients with reduced left ventricular dysfunction with or without a prior myocardial infarction, even in the absence of symptoms [25, 26]. Similarly, clinical studies on ACE-I suggest comparable effects in women and men with heart failure.
Angiotensin II receptor blockers (ARBs) are accepted as a potential alternative to ACE-I, especially in patients who are intolerant to ACE-I secondary to cough [27]. ARBs are non-inferior to ACE-I in the management of post-MI patients complicated by symptomatic heart failure [28]. Subsequent analysis of several heart failure trials have found a similar significant reduction in mortality and HF hospitalizations in women as compared to men [29]. The recommendations for ARBs are similar for men and women and are recommended for those intolerant to ACE-I.
Beta-blockers are also an accepted first line therapy for symptomatic heart failure patients. The mechanism of benefit from beta-blockers in heart failure is thought to involve inhibition of the long-term deleterious effects of the sympathetic nervous system. In patients with HFREF, beta-blockers have been found to significantly decrease mortality and heart failure re-hospitalizations [30–32]. Review of the beta-blocker data on women with symptomatic HFrEF suggests that both genders receive similar benefit from the use beta-blockers [33]. Given this evidence, recommendations are to use beta- blockers for both men and women with heart failure.
Data from the African-American Heart Failure Trial (A-HeFT) demonstrated a reduction in mortality of 43 % using hydralazine and isosorbide dinitrate in addition to standard therapy, including ACE-I and beta blockers [34]. The A-HeFT trial included 40 % women, more than previous trials, and found similar improvement in heart failure outcomes including primary composite score, first heart failure hospitalization, and event-free survival in both men and women [35].
Sustained activation of aldosterone appears to play an important role in the pathophysiology of heart failure with elevated circulating levels of aldosterone enhancing sodium retention. In addition, deleterious effects on the vasculature and myocardium have been documented [36]. There is a general lack of sex-specific data from prospective trials on the benefits of aldosterone antagonists for women with left ventricular systolic dysfunction and symptoms of heart failure. However, adequate numbers of women were included in two large randomized, controlled trials of these agents and subgroup analyses were shown to demonstrate benefit in women [37, 38]. Similar to prior HF guidelines, the most recent 2013 ACC/AHA guidelines offer safeguards of creatinine levels needing to be ≤2.0 mg/dL in women (vs. 2.5 mg/dL in men) [10].
Digoxin therapy, though demonstrated to decrease heart failure hospitalizations, has not been shown to improve survival [39]. Retrospective analysis of the Digitalis Investigation Group (DIG) trial suggested an increased risk of death from any cause among women, but not men, with heart failure and reduced left ventricular ejection fraction [40]. However, further analysis of the same trial reported no excess mortality in either women or men when serum digoxin concentrations were between 0.5 and 0.9 mg/ml [41]. Digoxin levels are higher in women compared to men at any given dose likely due to decreased lean body mass and renal function.
Management of HF with Preserved Ejection Fraction (HFpEF)
HFpEF is associated with increased morbidity and mortality similar to that seen in the population with reduced ejection fraction [4, 5]. This is especially concerning for women, since females are more likely to have HFpEF than their male counterparts [6]. Unlike the clinical trial data in the HFrEF population, women have been well represented in the clinical trials of HFpEF. Among the few prospective studies of HFpEF, the percentage of females ranged from 61 to 76 % of the overall study populations [3, 42, 43]. Several professional societies have published guidelines that specifically address HFpEF [1, 44]. However, none of these guidelines, including the most recent 2013 ACC/AHA HF guidelines recently released [10] can be considered evidence-based, as there are currently no proven heart failure-specific therapies for which to use in this population, unlike those with reduced left ventricular ejection fraction. Society recommendations are generally similar and include the use of long-term diuretic therapy, when appropriate, to control or prevent edema along with ACE-I, ARBs and beta-blockers to treat hypertension (Table 8.4). Low sodium diet, without specific dietary sodium restriction (previously advocated on 2005 ACC/AHA guidelines), and fluid restriction recommendations are similar to that of the HFrEF population. Evaluation for ischemic heart disease and inducible myocardial ischemia, when applicable is also recommended [1, 44].
Table 8.4
Recommendations for treating patients with heart failure and preserved left ventricular ejection fraction
Class I guidelines |
Physicians should control hypertension in accordance with published guidelines (Level of Evidence B) |
Physicians should use diuretics to control pulmonary congestion and peripheral edema (Level of Evidence C) |
Class IIa guidelines |
Coronary revascularization is reasonable in patients with CAD in whom ischemia is having adverse effect on cardiac function despite GDMT (Level of Evidence C) |
Physicians should manage atrial fibrillation according to guidelines to improve symptoms of HF (Level of Evidence C) |
Beta blockers, ACE inhibitors, and Angiotensin II receptor blockers should be used for BP control (Level of Evidence C) |
Class IIb guidelines |
Angiotensin II receptor blockers may be useful in decreasing hospitalizations (Level of Evidence B) |
Class III guidelines |
Nutritional supplementation is not recommended in HFpEF (Level of evidence C) |
Cardiac resynchronization therapy with defibrillator (CRT-D) is an approved treatment for patients with advanced stages of heart failure in the setting of a widened QRS, and this therapy is associated with a reduction in symptoms, improvement in functional capacity, and a decrease in hospitalization and mortality [45]. Data suggests that women seem to benefit more from CRT-ICD therapy than men. As a result of CRT-D therapy, women have decreased cardiac volumes with improvements in LVEF compared to males [46]. In addition, a sub-study of the MADIT-CRT Trial demonstrated that women may have a significantly greater benefit from device therapy than men with regards to overall mortality and cardiac-related outcomes [47]. The reason for this seemingly sex-related benefit is unclear but may relate to the higher proportion of studied males with ischemic cardiomyopathy and non-left bundle branch block morphology on ECG, both indicators of poor response to CRT-D [47].
Takotsubo’s Cardiomyopathy
Takotsubo’s cardiomyopathy (TC), also referred to as the apical ballooning or broken heart syndrome is a rare disorder first described in the early 1990s by the Japanese. They reported on a unique, reversible cardiomyopathy that appeared to be precipitated by acute stress [48]. They discovered that these subjects were usually postmenopausal women who often developed signs and symptoms of an acute coronary syndrome (ACS) proximate to a strong emotional stressor. This presentation was associated with a transient apical and/or mid-ventricular wall motion abnormality resembling an octopus trap [48] (Fig. 8.2).
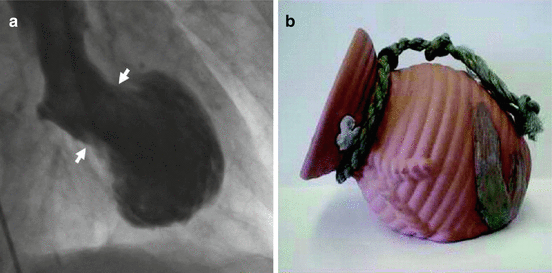

Fig. 8.2
(a) Ventriculogram of the heart during the contraction phase from a patient with takotsubo’s cardiomyopathy. Note the distinctive shape with a narrow neck (arrows) and ballooned lower portion, which contracts abnormally. (b) The Japanese takotsubo (ceramic pot used to trap octopus) has a shape that closely resembles that of the heart on the left. (Reproduced with permission from Sharkey [89])
Subsequent angiography typically displayed a lack of obstructive coronary artery disease (CAD).
The precise pathophysiologic mechanism of TC is unknown. Pharmacologically induced coronary artery spasm has been documented in a number of TC cases [49]. However, the actual significance of these clinical findings remain unclear at the present time. The presence of a catecholamine surge has accrued perhaps the most evidence in favor of a mechanism for this cardiomyopathy [50]. TC typically occurs proximate to either a physical or emotional stressor, states characterized by high circulating catecholamines. Finally, animal studies have shown that the administration of both alpha and beta blocker therapy prevents the development of this form of cardiomyopathy [51].
To date, it is largely unknown why TC predominates in women but several theories have been proposed to explain this finding. One possibility may be related to gender differences in myocardial sensitivity to catecholamine toxicity with subsequent intra-myocyte calcium overload [52]. Another is that females typically have a smaller left ventricular cavity size which may predispose them to left ventricular outflow tract obstruction and increased intra-ventricular pressure gradients. This may in turn cause an oxygen mismatch in the apex resulting in ballooning [53].
As mentioned previously, those presenting with TC are most commonly postmenopausal women [48]. In a systematic review, women accounted for 82–100 % of patients with an average age of 62–75 years, although cases have been described in individuals as young as 10 and as old as 91 years of age [54]. Typically, a prior strong emotional or physical stressor predates presentation though in some no precipitating event is identified [48]. Clinical presentation usually involves acute dyspnea and chest pressure or tightness not dissimilar to that of a patient with an acute coronary syndrome. ECG abnormalities are a mainstay and include ST elevation, pathologic appearing Q waves, QT prolongation and deep, symmetrical T wave inversions [54] (Fig. 8.3).
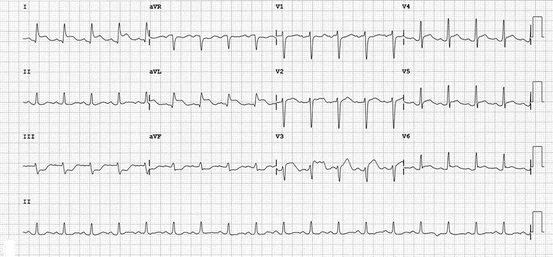

Fig. 8.3
Electrocardiogram of a patient with takotsubo’s cardiomyopathy demonstrating ST segment elevation in the anterior precordial and high lateral limb leads with recipricol changes in the inferior limb leads. (Reproduced with permission from Sharkey [89])
These findings, when combined with cardiac biomarker elevations that are commonly seen make it very difficult on initial evaluation to distinguish TC from that of an ST elevation myocardial infarction. Furthermore, it has been estimated that as many as 6 % of women presenting with an apparent ACS may actually have TC [55]. The hallmark echocardiographic findings consist of apical and/or midventricular wall motion abnormalities that do not correlate with the distribution of a single major epicardial coronary distribution with hyperkinesis of the basal myocardial segments [54]. Variants have been reported in young females presenting with stress cardiomyopathy after an acute emotional or physical stress demonstrating isolated basal hypokinesis only [55] (Fig. 8.4)
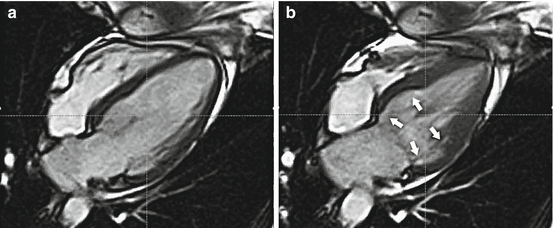

Fig. 8.4
Cardiac magnetic resonance imaging demonstrating in (a) diastole and (b) systole. The unusual pattern of basal akinesis (arrows) with preserved mid and apical function. The so-called “reverse takotsubo pattern”. (Reproduced with permission from Sharkey [89])
Emergent cardiac catheterization most commonly reveals a lack of obstructive coronary artery disease that could account for the observed regional wall motion abnormalities.
Currently there is a lack of available clinical trial data in TC with which to guide therapy so management is typically performed on an empiric basis. Fortunately, due to the reversible nature of TC, supportive care will usually suffice. In rare instances, hemodynamic compromise leading to cardiogenic shock occurs, requiring the assistance of intravenous inotropes and even mechanical support by means of an intra-aortic balloon pump. Anticoagulation should be considered, at least during the initial hospital course to prevent the formation of left ventricular thrombus and subsequent thromboembolism, which is a common cause of mortality in these patients. Given the role that catecholamines may play in the pathophysiology of TC, long-term beta-blocker therapy to prevent recurrence has been suggested though no clear and consistent data exists to support its use [54].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


