Heart Failure
Gregg C. Fonarow
Heart failure (HF) affects 5 million Americans, and 550,000 new diagnoses are made each year (1). HF results in approximately 1 million hospitalizations, which translates into an annual estimated cost of nearly $23 billion. Prognosis is poor once a patient has been hospitalized with HF; the mortality risk after HF hospitalization is 11.3% at 30 days, 33.1% at 1 year, and well over 50% within 5 years (2). Heart failure is also the only major cardiovascular condition that is increasing in both its incidence and prevalence, which places a significant burden on the health care system. The almost 1 million hospital discharges due to HF in 2002 represents a 152% increase since 1972. Therefore, both high risks and costs are associated with HF hospitalization (3,4,5). In addition to the risk of mortality, patients diagnosed with HF have an increased risk of rehospitalization. In a study of almost 18,000 Medicare recipients, approximately 44% were rehospitalized at least once in the year following their index hospitalization. Nearly 20% of patients are rehospitalized twice for the same condition (6), and a number of studies indicate that a significant proportion of rehospitalizations for HF appear to be preventable (7,8,9). These statistics emphasize the need to develop and implement strategies to improve HF care.
Underutilization of Standard-of-Care Therapies in Heart Failure
Prior studies have shown that evidence-based, guideline-recommended therapies are significantly underutilized, and there is substantial variation in adherence to performance measures among hospitals caring for patients with HF (10,11,12,13,14). A well-documented treatment gap exists between national HF guidelines and the clinical management of HF. This HF treatment gap results from a variety of complex issues, including a lack of systems and disease management programs.
Despite the favorable effect on morbidity and mortality that has been demonstrated by the use of angiotensin-converting enzyme (ACE) inhibitors, studies indicate that only 48% of patients with previous HF were receiving these agents at the time of hospitalization (13), and that only 60% to 70% of eligible HF patients received ACE inhibitors at discharge (15,16). The Acute Decompensated Heart Failure National Registry (ADHERE) reported a similar statistic, with only 47% of chronic HF patients with previously diagnosed systolic dysfunction receiving a β-blocker on an outpatient basis before admission to the hospital (13).
Evidence-based clinical practice guidelines have been developed for the treatment of patients with HF (3,17,18,19), and components of these guidelines have been adapted by the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) to create core performance measures for patients hospitalized with HF (Table 20-1) (20,21,22).
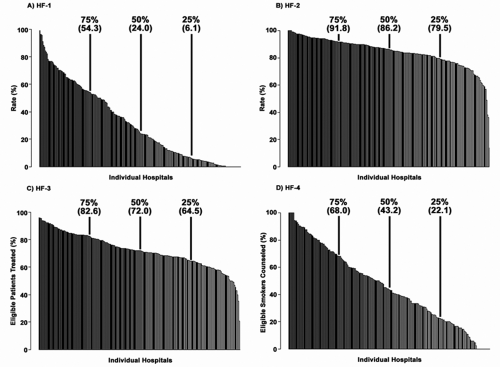
However, adherence to JCAHO core measures is variable. Data from more than 80,000 HF admissions to academic and nonacademic hospitals in the United States participating in the ADHERE registry were analyzed to determine the rates of conformity with the four core performance measures: discharge instructions (HF-1), assessment of left ventricular function (HF-2), use of ACE inhibitors in patients with left ventricular systolic dysfunction (LVSD) (HF-3), and smoking cessation counseling (HF-4) (23). Across all hospitals, the median rates of conformity with HF-1, HF-2, HF-3, and HF-4 were 24.0%, 86.2%, 72.0%, and 43.2%, respectively. Rates of conformity at individual hospitals varied from 0% to 100%, with statistically significant differences between academic and nonacademic hospitals. Figure 20-1 depicts the frequency distribution of conformity rates by hospital (23). For each measure, there were substantial clinically relevant differences in performance between hospitals at different percentile levels. These differences were most notable for the measures with the lowest overall conformity. For HF-1, there was a 100-fold difference in conformity between hospitals at the 10th and 90th percentiles. For HF-4, there was an 11.2-fold difference in conformity at these percentiles. In contrast, there was a 1.3- and a 1.5-fold difference in conformity between hospitals at the 10th and 90th percentiles for HF-2 and HF-3, respectively (23).
Median hospital length of stay varied from 2.3 to 9.5 days, and in-hospital mortality varied from 0% to 11.1%. Among the hospitals enrolled in ADHERE, there was significant individual variability in conformity to quality-of-care indicators and clinical outcomes and a substantial gap in overall performance. Academic and nonacademic hospitals differed in their conformity with the four performance measures. Nonacademic hospitals demonstrated significantly better median conformity with HF-1 than academic hospitals, whereas academic hospitals demonstrated slightly better median conformity than nonacademic hospitals with HF-2 and HF-3 (23).
The gaps in care that currently exist in the hospital suggest that new efforts targeting education and effective patient
intervention should be investigated to improve the overall quality of care for patients with HF. Hospital-based systems for HF have been demonstrated to improve the provision of evidence-based care. Previous studies have demonstrated the importance of hospital-based systems and in-hospital initiation of cardiovascular protective therapies for improving treatment rates and clinical outcomes in patients with atherosclerosis (12,24).
intervention should be investigated to improve the overall quality of care for patients with HF. Hospital-based systems for HF have been demonstrated to improve the provision of evidence-based care. Previous studies have demonstrated the importance of hospital-based systems and in-hospital initiation of cardiovascular protective therapies for improving treatment rates and clinical outcomes in patients with atherosclerosis (12,24).
Table 20-1. Jcaho Core Performance Measures for Heart Failure | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
In-Hospital Initiation of Therapy
The setting in which HF therapy is initiated is an important factor influencing treatment and compliance/adherence rates. The care of HF patients while hospitalized is critical to their outcome. Therapy that is initiated in the hospital is likely to
have higher adherence rates due to both patient and physician behavior: the hospital is a patient “capture point.” Therapy recommended in the hospital carries more weight with the patient and will be viewed as a necessary, lifesaving medication. The hospital setting also provides a teachable moment, where the physician has the attention of both the patient and family members. Other physicians, nurses, and health care workers are also available to answer questions. The hospital structure can also provide a better setting for initiation of therapy, because the patient is monitored closely, and the physician uses standard protocols and guidelines for treatment.
have higher adherence rates due to both patient and physician behavior: the hospital is a patient “capture point.” Therapy recommended in the hospital carries more weight with the patient and will be viewed as a necessary, lifesaving medication. The hospital setting also provides a teachable moment, where the physician has the attention of both the patient and family members. Other physicians, nurses, and health care workers are also available to answer questions. The hospital structure can also provide a better setting for initiation of therapy, because the patient is monitored closely, and the physician uses standard protocols and guidelines for treatment.
The importance of initiating therapy before a patient is discharged cannot be overemphasized. The implementation of comprehensive in-hospital HF disease management programs, which include management of medications, patient education, and medical staff guidelines, results in improvements in medication dosing and decreased hospitalization for HF patients (12). The Duke Heart Failure Program, for example, showed a significant increase in both the use of β-blockers and the percentage of patients reaching target doses of β-blockers and a decrease in cost of care throughout the hospital system (12).
Various hospitals have developed a number of tools to encourage physicians to initiate as many lifesaving therapies as possible before patient discharge. The use of preprinted orders, care maps, discharge forms, physician/nursing education, and treatment utilization reports may facilitate the implementation of therapy. These tools are designed to help health care providers identify and initiate guideline-recommended treatments in appropriate HF patients without contraindications. The tools also provide guidance in identifying and managing factors that may exacerbate HF, major comorbidities, and concomitant risk factors (e.g., sudden death risk, lipids, diabetes, anemia, chronic obstructive pulmonary disease, and depression). These critical pathways also aim to enhance patient education, assessment of social support, discharge planning, follow-up, and outpatient monitoring.
Risk-Treatment Mismatch
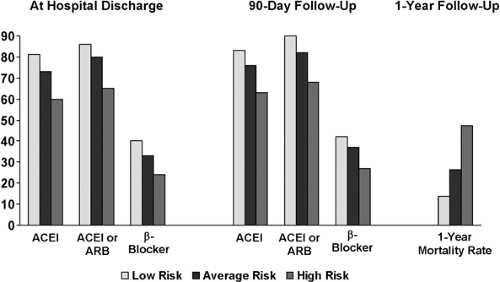
Unfortunately, there is a risk-treatment mismatch in HF: patients with severe HF or those with the highest risk of morbidity and mortality are less likely to receive therapy than those with lower risk. In a recent study of HF drug administration rates at hospital discharge and 90 days after discharge, the highest-risk HF patients were much less likely to receive lifesaving therapies (25). Low-risk patients were more likely to receive ACE inhibitors or angiotensin-receptor blockers (ARBs) (adjusted hazard ratio [HR], 1.61; 95% confidence interval [CI], 1.49 to 1.74) and β-receptor antagonists (HR, 1.80; 95% CI, 1.60 to 2.01) compared with high-risk patients (both P <0.001) (Fig. 20-2) (25).
The highest-risk patients, those who have the greatest risk of death due to comorbidities, the lowest ejection fraction (EF), and other indicators of poor outcomes during hospitalization for HF, are the ones who would derive the greatest benefit from treatment. Because drug administration is currently inversely associated with risk, the potential benefit of HF pharmacotherapy will not be realized if current patterns continue. Greater quality improvement efforts aimed at increasing use of HF drugs in higher-risk patients are needed (25).
In addition, recognition of the patients at highest risk is important to the use of optimal therapy. Achieving the most accurate estimation of risk for poor outcomes after patients have been hospitalized for HF may help clinicians guide the type and intensity of therapy. Recent registries and clinical trials have identified markers of poor outcomes in hospitalized HF patients.
The Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) study found that in 949 patients with decompensated HF, the variables at presentation that predicted death at 60 days were older age, lower systolic blood pressure, New York Heart Association (NYHA) class IV symptoms, elevated blood urea nitrogen (BUN), and decreased sodium (26). In a study of 988 patients with HF and preserved EF enrolled in the Digitalis Investigation Group (DIG) trial, among 18 variables considered, the strongest independent predictors of death were glomerular filtration rate, NYHA functional class III or IV, male gender, and older age (27).
The ADHERE program of more than 30,000 patients developed and validated a practical and user-friendly method of risk stratification for in-hospital mortality among patients
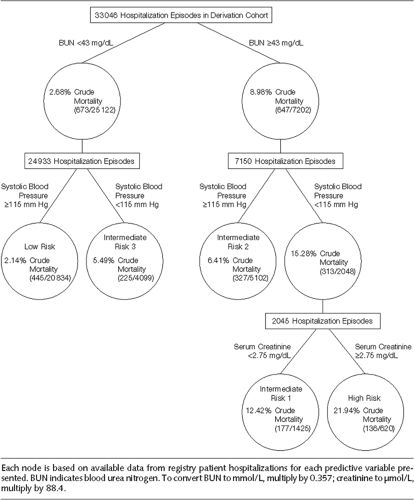
admitted with HF that could be applicable to the bedside (28). High-risk patients could be identified to determine in which patients resource-intensive interventions designed to improve outcomes may be justified. Of 39 variables in ADHERE, the following were independent predictors of high risk for in-hospital mortality in classification and regression tree (CART) analysis: BUN level of 43 mg/dL or higher, serum creatinine level of 2.75 mg/dL or higher, and systolic blood pressure (SBP) of less than 115 mm Hg. In this multivariate analysis, blood pressure (BP), heart rate, serum creatinine, serum sodium, and liver disease were highly predictive of in-hospital mortality. Acutely decompensated HF patients at low, intermediate, and high risk for in-hospital mortality can be easily identified using vital signs and laboratory data obtained on hospital admission. The ADHERE risk tree provides clinicians with a validated, practical bedside tool for mortality risk stratification (Fig. 20-3) (28).
admitted with HF that could be applicable to the bedside (28). High-risk patients could be identified to determine in which patients resource-intensive interventions designed to improve outcomes may be justified. Of 39 variables in ADHERE, the following were independent predictors of high risk for in-hospital mortality in classification and regression tree (CART) analysis: BUN level of 43 mg/dL or higher, serum creatinine level of 2.75 mg/dL or higher, and systolic blood pressure (SBP) of less than 115 mm Hg. In this multivariate analysis, blood pressure (BP), heart rate, serum creatinine, serum sodium, and liver disease were highly predictive of in-hospital mortality. Acutely decompensated HF patients at low, intermediate, and high risk for in-hospital mortality can be easily identified using vital signs and laboratory data obtained on hospital admission. The ADHERE risk tree provides clinicians with a validated, practical bedside tool for mortality risk stratification (Fig. 20-3) (28).
Critical Pathway Tools
The continued persistence of suboptimal compliance in all hospitals and the significant variability between hospitals in both compliance and outcome variables provide a compelling rationale for the implementation of critical pathway tools that may improve hospital performance.
It is important to recognize why therapy is suboptimal in order to devise effective strategies to improve it. Some of the barriers that prevent the implementation of cardioprotective therapies in patients with HF are time constraints; lack of physician training, including inadequate appreciation of benefits and lack of prescription experience; a lack of resources; and lack of communication between specialists and generalists. In addition, the adherence to old guidelines that call for delaying initiation of therapy and including multiple steps, time points, and too many treatment options may deter physicians from using optimal care. The use of HF critical pathway tools that simplify care and clarify the roles of each caregiver may break down some of these barriers. Use of these tools may also eliminate the variability in care between hospitals and improve the overall treatment of hospitalized HF patients. The structure and education that these tools provide, in addition to improving coordination of care among staff in the hospital, make process-of-care programs invaluable.
Process-of-care programs are designed to implement a number of tools to check the treatment of the hospitalized HF patient at various stages, from admission, throughout hospital stay, and at discharge. An example of an HF admission checklist is shown in Figure 20-4. This tool will aid in diagnosis, prompt the appropriate laboratory work, and remind the physician of the importance of an echocardiogram in HF patients to evaluate EF. Use of this checklist can help the physician more accurately assess a patient’s needs for indicated therapy as well as facilitate identification of patients with contraindications to one or more therapies.
An example of standardized orders for HF is shown in Figure 20-5. This easy-to-read form serves as a guide for the physician, nurse, and other caregivers and allows each of them to follow standard HF treatment protocols and indicate each guideline-recommended therapy that has been initiated. These preprinted order sets can facilitate the identi-fication of patients with contraindications to one or more therapies and thus enhance patient safety, provide standard medication dosing, emphasize the need for monitoring certain laboratory tests, and help avoid medical errors of omission. The sets can also facilitate consultation and collaborative care.
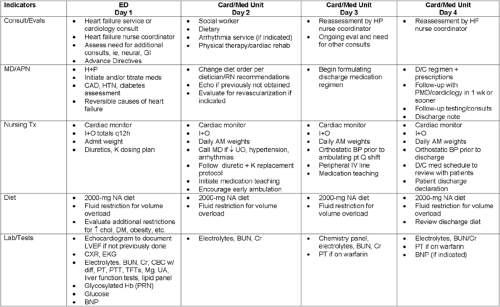
In addition, a comprehensive HF critical pathway tool may be used throughout the patient’s hospitalization to monitor care (Fig. 20-6). This tool may help the physician recognize the signs of poor outcomes, higher in-hospital mortality, and rehospitalization. Deviations from the critical pathway
can be documented and later analyzed to better understand variations and patterns to facilitate the quality improvement process.
can be documented and later analyzed to better understand variations and patterns to facilitate the quality improvement process.
In addition to all the tools contained in this chapter, a number of critical pathway aids should be used in all hospitals to improve the quality of care. Algorithms, care paths, standing orders, and patient education materials can all aid the physician in the hospital. A discharge summary checklist may be an important component, as it forces physicians to evaluate the therapy on which a patient is discharged and—in light of data that show increased adherence to medications that are prescribed before discharge—may cause them to initiate additional therapy (Fig. 20-7). As discussed previously, patients that are discharged on therapy are more likely to remain on therapy.
Success of Critical Pathways
The benefits of a hospital-based system to improve the use of ACE inhibitors before hospital discharge were demonstrated by an analysis of more than 19,000 patients discharged after an HF-related hospitalization at a ten-hospital integrated health care system (29). Comparisons were made between patients discharged before (n = 11,038) and after (n = 8,045) the implementation of an HF discharge medication program. As a result of the program, the use of ACE inhibitors increased from 65% to 95%. The program also resulted in a reduction in the rate of readmission from 46.5% to 38.4% (P <0.0001) and in the rate of mortality from 22.7% to 17.8% (P <0.0001) at 1 year (29). Similar significant improvements were seen in a comprehensive HF management program involving 214 patients (NYHA class III or IV) who were candidates for heart transplantation (24). The program included modifications in drug therapy, patient education, and regular follow-up with the HF team. When data were compared for the 6-month period before initiation of the program with those from the 6 months following implementation, it was found that ACE inhibitor use increased by 18% (from 77% to 95%, P = 0.05) and hospital admission declined by 85% (P <0.0001) (24).
The Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy for Heart Failure (IMPACT-HF)
was a multicenter, open-label trial involving 363 HF patients with left ventricular ejection fraction (LVEF) ≤40% who were admitted for an episode of HF decompensation (30). Patients were randomized to receive initiation of carvedilol therapy before hospital discharge (once the HF decompensation was treated successfully) or to usual care (initiation of any β-blocker 2 to 4 weeks after hospitalization and when the patient became stable). At the end of 60 days, 91% of the predischarge initiation patients were on carvedilol; in contrast, only 73% of patients in the postdischarge, physician-discretion initiation group (P <0.0001) were receiving any β-blocker. In the carvedilol initiation group, the mean percentage of target dose achieved was 36.3%; in the postdischarge initiation arm, it was 28.6% (P = 0.02). The median length of stay for patients did not increase with predischarge initiation, and there was no increased risk of serious adverse events (30).
was a multicenter, open-label trial involving 363 HF patients with left ventricular ejection fraction (LVEF) ≤40% who were admitted for an episode of HF decompensation (30). Patients were randomized to receive initiation of carvedilol therapy before hospital discharge (once the HF decompensation was treated successfully) or to usual care (initiation of any β-blocker 2 to 4 weeks after hospitalization and when the patient became stable). At the end of 60 days, 91% of the predischarge initiation patients were on carvedilol; in contrast, only 73% of patients in the postdischarge, physician-discretion initiation group (P <0.0001) were receiving any β-blocker. In the carvedilol initiation group, the mean percentage of target dose achieved was 36.3%; in the postdischarge initiation arm, it was 28.6% (P = 0.02). The median length of stay for patients did not increase with predischarge initiation, and there was no increased risk of serious adverse events (30).
The Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) is the first national, hospital-based program designed to improve medical care and education of hospitalized HF patients and to accelerate the use of evidence-based, guideline-recommended therapies by in-hospital initiation (31). OPTIMIZE-HF has two main components designed to achieve its objectives: a Web-based registry and a process-of-care improvement plan. The registry tracked the use of lifesaving therapies before and after initiation as well as hospital progress and discharge planning. The Web site provided real-time reports and benchmark comparisons among institutions, both regionally and nationally, allowing them to share best practices. The registry also captured outcome data for patients at 60 to 90 days after enrollment. Data on the use of ACE inhibitors and angiotensin-receptor antagonists in systolic HF patients were collected, as were data on the use of evidence-based β-blockers. This provided hospitals with a greater ability to understand practice patterns and current quality of care than they would have obtained using a limited number of static performance measures.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree