Implant Cost
REMATCH
HeartMate II DT Trial
p-Value
N
54
98
<0.01
Cost ($)
384,260 ± 340,456
193,812 ± 71,027
Median ($)
245,445
186,156
Heart transplant without an LVAD bridge is the most cost-effective course, but the sickest patients often cannot continue waiting without circulatory support. BTT with transplant requires two major operations with considerable hospital time for both procedures, expectedly making this approach costly [29]. Studies have identified reimbursement for LVAD support as inadequate when compared to transplant; however, third-party payer reimbursement has improved along with better survival making BTT more cost-effective [30, 31]. In a more recent head-to-head cost comparison of heart transplant to LVAD support at 1 year, the average cost was approximately $40,000 high for LVAD support, but this difference was not statistically significant [32]. Comparison of LVAD support to heart transplant is problematic since patients undergoing LVAD implant for BTT are likely to be much sicker or are at higher risk of death than patients undergoing elective transplant. Furthermore, LVAD support in the sickest transplant candidates optimizes organ function and physical status lowering the risk of post-transplant complications. Cost-effective comparisons inherently give transplant an advantage [33].
In the Center for Medicare Medicaid Services, Medicare Provider Analysis and Review (MEDPAR) file contains data from claims for services provided to beneficiaries admitted to Medicare certified inpatient hospitals. MEDPAR 2013 hospital cost reported the volume, length of stay (days), and average cost for implanting an LVAD and heart transplantation (◘ Table 57.2). The hospital cost for implanting an LVAD, which includes the additional expense of the device and accessories, is becoming similar to heart transplantation cost.
Table 57.2
Comparison of heart transplant and implantable assist device
MS-DRG 1 case mix | Case volume (N) | Average length of stay (days) | Average calculated cost ($) | Average organ acquisition cost ($) | Average total cost ($) |
|---|---|---|---|---|---|
Heart transplant (ICD-9 Px 33.6 and 37.51) | 648 | 32 | 116,265.71 | 28,406.14 | 144,673 |
Implantable heart assist (ICD-9 Px 37.52 and 37.66) | 1259 | 34 | 196,595.26 | Not applicable | 196,026 |
The UK National Institute of Health Research, analyzing the clinical effectiveness and cost-effectiveness of LVADs as BTT, completed a systematic review and cost-effectiveness model [37]. The aims of this study were to determine the clinical effectiveness and cost-effectiveness of continuous-flow LVADs used for BTT versus medical therapy and BTT versus destination therapy. The systematic review included devices approved for BTT by the US Food and Drug Administration and Conformité Européenne (CE) approved – the two systems with results were the HeartMate II and the HVAD. Forty publications were identified to provide the outcome data, while cost-effectiveness data was gathered from implants performed at six institutions in the United Kingdom. There were no randomized controlled trials in the analysis. A semi-Markov model with multiple sensitivity analyses varying survival, utilities, and cost inputs to the model was used. The model outputs were incremental cost-effectiveness ratios (ICERs), cost-/quality-adjusted life-years (QALYs) gained and cost/life-year gained (LYG). They reported that the 3-year, 10-year, and lifetime ICERs for BTT with an LVAD compared to medical management are higher than generally applied to willingness-to-pay thresholds. Nonetheless, at a lifetime time horizon, the ICERs approximate the threshold values used in end-of-life assessments. They had determined that LVADs for BTT yields ICERs of £122,730, £68,088, and £55,173, respectively, when compared with medical management.
At a lifetime horizon, using VADs as an alternative to transplant (ATT) rather than as a BTT was complex and had a reduced cost and reduced quality-adjusted life-years (QALYs). ATT when evaluated over a lifetime was £20,637 less costly than BTT for each QALY year. An important conclusion from this study was the need of published studies that include cost with survival, quality of life, functional capacity, and adverse event rates.
During the HeartMate II clinical trial, a Markov model was developed to assess cost-effectiveness of DT as compared to medical management [38]. Survival, hospitalization rates, quality of life, and cost data were analyzed for advanced heart failure patients treated with LVAD support or medical therapy. Clinical outcomes were obtained from the medical therapy arm of the REMATCH trial and from patients treated with an LVAD in the HeartMate II destination therapy clinical trial. The cost of heart failure admissions was estimated with Medicare prospective payments, and the cost of LVAD implantation was obtained from hospital claims during the clinical trial. Compared to patients treated with optimal medical therapy, continuous-flow LVAD patients had higher 5-year costs ($360,407 versus $62,856), QALYs (1.87 versus 0.37), and life-years (2.42 versus 0.64). The ICER of the continuous-flow device was $198,184 per QALY and $167,208 per life-year. This equates to a 75% reduction in ICER compared to the $802,700 per QALY for the pulsatile-flow device. The results were most sensitive to the cost of device implantation, long-term survival, cost per re-hospitalization, and utility associated with patients’ functional status. This study concluded that cost-effectiveness of LVAD support was improving for patients treated for destination therapy with the newer continuous-flow LVAD. These results were attributed to better survival, lower costs of implantation, and better functional capacity of supported patients. Although the ICER/QALY was higher than the conventional $50,000, the high rate of cost reduction was very encouraging. The observed 75% reduction in QALY/year occurred during a time when LVAD implants were increasing substantially with the anticipation of further improvements. The findings and conclusions of this study were at a time when the use of continuous-flow LVADs was relatively early, and with clinical experience, efficiency and survival are likely.
57.2 Evolving Cost-Effectiveness: How It Is Changing
A number of cost-effectiveness studies have been performed with retrospective data on patients supported with the older generation of pulsatile LVADs [39–41]. These studies conclude that improvements in LVAD technology are necessary to improve cost-effectiveness, and continued reassessment is desired. Over the past decade, there has been a continual improvement in outcomes of durable continuous-flow LVAD support, with 6-month and 1-year survival rates for BTT near 94% and 85%, respectively [42]. This compares to the early results, which showed rates of 75% at 6 months and 68% at 1 year [43]. For sicker patients supported for DT, survival at 1 year has increased from 68 to 74%, and at 2 years, the increase was 58–61% [44]. These improvements are largely due to better timing of LVAD implant, preoperative optimization, postoperative management protocols, and refinements in the technology [45]. The overall rate of serious adverse events has steadily declined, which has a significant impact on hospitalization time, the number of interventions, and the total cost of care. Therefore, overall effectiveness of LVAD therapy is continuously evolving in a positive direction, but the longer survival time increases overall cost and offsets some of this gain [46, 47].
The evolution of LVAD therapy for advanced heart failure has been unique when compared to other devices and pharmacological therapy [48]. The change from pulsatile- to continuous-flow LVAD technology was relatively rapid as clinicians identified the benefits of the smaller and more durable devices. The “learning curve” associated with the application of the continuous-flow devices was relatively short, and outcomes have improved steadily over the 15 years since their inception [42]. In this same time frame, the ICER for pulsatile LVADs during the REMATCH trial was $602,361, which then decreased to $187,989 in the post-trial time and then to $107,569 with the use of the current continuous-flow devices (◘ Fig. 57.1) [48]. This level of ICER nears the $100,000 mark of willingness to pay per life-year saved. This progress in cost-effectiveness is compelling, yet controlled unbiased data from clinical trials that guides policy decisions is needed. Furthermore, because LVAD technology is constantly evolving and patient care improves with experience, cost-effectiveness analysis also needs to be continuous and its methods developed.
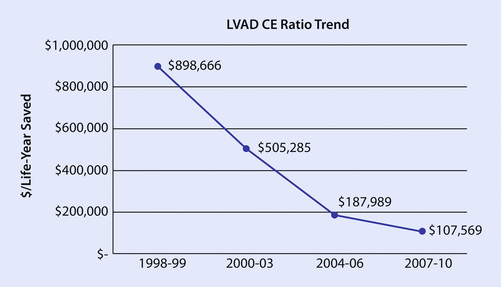

Fig. 57.1
LVAD CE ratio over time
Although LVAD support for both BTT and DT extends survival and the majority of patients experience improved quality of life, current cost-effectiveness studies indicate that further reduction in adverse events is necessary for this therapy to exceed cost-effectiveness thresholds [49–51]. Re-hospitalization and follow-up care for both BTT and DT indications are significant drivers for the high cost of LVAD support [52]. In particular, gastrointestinal bleeding (GIB) and infection are the leading causes for readmission, with the median cost of each readmission at approximately $7500 [53]. Unfortunately, GIB is very common with the current generation of continuous-flow LVADs, and it is currently unknown how to avoid this complication. Infection readmission is also common and is one of the more costly adverse events seen in the LVAD population. Less frequent, yet costly events occurring over the course of LVAD support are pump thrombosis requiring pump exchange and stroke. Methods for reducing device-related adverse events, particularly driveline infection, may prove to have a positive impact in reducing costs. Careful monitoring of outpatients and further development of shared-care resources may help to identify problems sooner, allowing for more effective diagnosis and treatment [54, 55].
57.2.1 Discussion
One of the past limitations for the use of LVADs was the requirement by protocol to implant these devices in the sickest patients whose chance of having a positive outcome was already limited. Since the commercialization of the continuous-flow LVAD, outcome measures have steadily improved while costs have remained fairly constant. Following clinical trials with the continuous-flow LVADs, there was a rapid expansion in the use of these devices worldwide. Indeed, some inexperienced centers had to pass through the “learning curve” before outcomes were at par with long-established centers where the clinical trials were conducted. Interestingly, centers without extensive experience in implanting LVADs can achieve excellent outcomes without high morbidity [56].
Early results from a HeartMate III clinical trial showed there were no instances of device exchange, pump thrombosis, or hemolysis [22]. With this reduction in adverse events, the HeartMate III may further reduce the cost of LVAD therapy. For patients who are ineligible for cardiac transplantation, this technology offers the clinician an increasingly cost-effective treatment option that prolongs survival and dramatically improves quality of life compared to traditional medical management. Progress made in device design and patient selection lowers cost and maximizes effectiveness of DT. With continued device-related improvements and clinical experience, LVAD implantation for DT will offer a cost-effective solution to heart failure patients ineligible for transplantation.
Published reports on LVAD therapy cost-effectiveness have come from the USA, United Kingdom, Italy, and Norway. Interpretation of the results in these studies must be done cautiously due to the considerable variability in the cost of delivering care and in the outcomes achieved. Larger controlled studies with well-defined methods are needed to better evaluate the cost-effectiveness of LVAD therapy. As LVAD technology improves and patient care experience progresses, better outcomes will be achieved at lower cost. Optimizing reimbursement requires a continuing assessment of this therapy.
57.3 Summary
Use of the LVAD for BTT and DT has evolved gradually, with results improving dramatically since these indications were first applied. Cost-effectiveness of LVAD therapy has progressed much, while further improvement is necessary to gain better acceptance from government agencies, third-party payers, and the general public. Clinical research must continue to further refine practices for reduced morbidity. Continued innovation by engineers should help to further refine device technology for optimizing biocompatibility and usability. Novel blood pump designs may offer improved biocompatibility, while clinicians must apply these devices in a cost-effective manner by selecting suitable candidates and employing standard practices.
References
1.
Cooley DA, Liotta D, Hallman GL, Bloodwell RD, Leachman RD, Milam JD (1969) Orthotopic cardiac prosthesis for two-staged cardiac replacement. Am J Cardiol 24:723–730CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


