Fig. 14.1
Home sleep recording showing ventilatory response to a disturbance (central apnea) in the setting of high loop gain chemoreceptor response. Notice how high loop gain results in overreaction to the disturbance and promotion of recurrent central apneas. A low loop gain response would result in progressive dampening of the subsequent respiratory event until normal respiration is recovered. From top to bottom: SpO2 (channels 1). Nasal airflow (channel 2). Thoracic effort (channel 3). Abdominal effort (channel 4)
Arousals from sleep also promote respiratory instability by producing hyperventilation , which in turn drives the PCO2 level below the apnea threshold. In the setting of high chemoreceptor sensitivity, this perpetuates respiratory instability and periodic breathing [30].
Normal sleep produces a relative state of hypoventilation, as noted by a 2–3 % reduction in SaO2 and by a 2–3 mmHg increase in PCO2 [33]. This degree of hypoventilation has no clinical relevance in normal individuals. However, in persons prone to having low lung volumes due to obesity, respiratory muscle weakness, or in those with intrinsic lung disease, this sleep-related hypoventilation may significantly contribute to central hypopneas and obstructive apneas, especially when sleeping in the supine position [34].
Sleep more than doubles upper airway resistance which can promote sleep-disordered breathing. This is the result of dramatic loss of inspiratory genioglossus motor unit activity at sleep onset resulting in the relaxation of the tongue and pharyngeal dilator muscles, causing a partial collapse of the upper airway [35]. Upper airway resistance is also greatly enhanced by gravity when sleeping in the supine position [36]. This effect can be most marked during the generalized muscle hypotonia of REM sleep. Clinically, it is common for snoring, which is a sign of increased upper airway resistance, to be most severe or present only when sleeping in the spine position.
Importance of Health Disparities in the Practice of Respiratory Sleep Medicine
The importance of sleep in overall health has only recently been recognized [23–25, 37–39]. Sleep-disordered breathing such as snoring and sleep apnea are widespread conditions [37, 40, 41] and are associated with adverse outcomes such as hypertension, diabetes, obesity, myocardial ischemia, stroke, arrhythmia, renal failure, increased healthcare use, and all-cause mortality [23–25]. Most of what is known about the epidemiology and pathophysiology of sleep-disordered breathing has come from research performed in predominantly male non-Hispanic white populations, with limited research in African Americans [37, 42–44]. Therefore, it is difficult to generalize the results to other ethnic minorities or to women. Differences in sleep architecture, duration, and sleep disorders have been reported between various racial groups [44–48]. In a study by Profant et al., African Americans had longer total sleep time, longer REM sleep, and lower deep sleep than whites [44]. In a more recent study from the same laboratory, African Americans had more N2 sleep (light sleep) and less deep sleep than whites, which was associated with a greater personal experience of discrimination as assessed using The Scale of Ethnic Experience [46]. In a review of the literature, children in racial/ethnic and socioeconomic minority groups had a higher prevalence and greater risk for sleep-disordered breathing [48]. This suggests significant environmental or cultural effects on sleep quality and sleep disorders that may impact the prevalence of cardiovascular disease and affect their evaluation and management [47, 48]. For example, children with health insurance who had OSA were more likely to undergo tonsillectomy to correct the problem than those without health insurance [48]. In 2003, the National Institutes of Health National Sleep Disorders Research Plan (NSDRP) stressed that there were major sleep health disparities in racial and ethnic minorities and in the socioeconomically disadvantaged, who are more likely to sleep in crowded, noisy, or otherwise less than optimal environments [49].
According to data from the 2010 US Census, minority groups in the US are rapidly growing, and by 2043, whites may become the minority. For example, Hispanics or Latinos are now the largest US minority group at 16.3 %; by 2050, it is estimated that Hispanics will make up 29 % of the US population [50]. These great shifts in demographics further stress the need to better understand health disparities and develop policies to help eliminate them.
Sleep-Disordered Breathing
Sleep-disordered breathing can be divided into obstructive (Fig. 14.2), central (Fig. 14.3), and mixed apneas (Fig. 14.4) and hypopneas. Apneas are characterized by complete or nearly complete cessation of airflow for at least 10 s in the adult and for two or more respiratory cycles in the child. Hypopneas are characterized by a 30 % to <90 % decrement in airflow for at least 10 s associated with oxyhemoglobin desaturation of ≥4 %. Alternatively, hypopneas can also be defined as a reduction in airflow of ≥50 % associated with an oxygen desaturation of ≥3 % and/or an arousal from sleep (Fig. 14.5) [51]. The severity of sleep apnea is characterized using the apnea–hypopnea index (AHI), which describes the number of these events per hour of sleep.
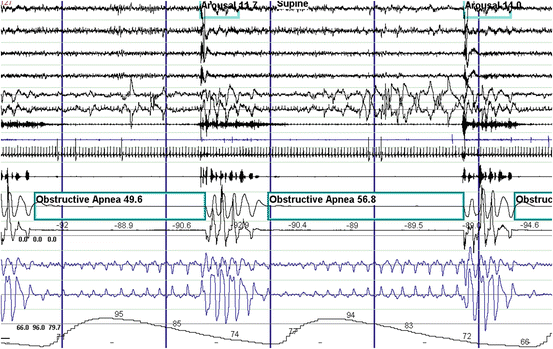
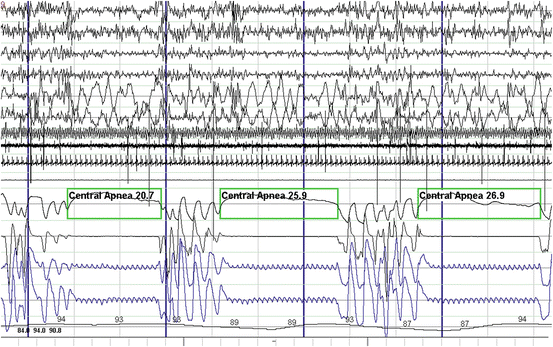
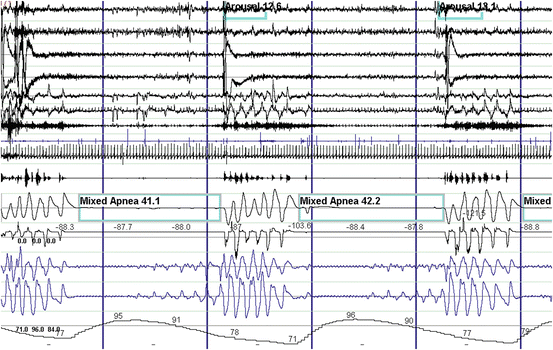
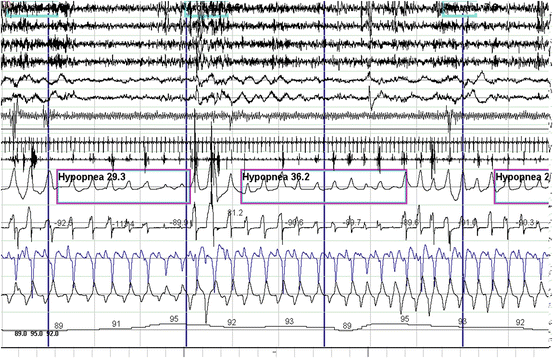

Fig. 14.2
Polysomnographic example of obstructive sleep apnea . These events are characterized by complete obstruction of the upper airway resulting in the absence of airflow while respiratory efforts persist. Gasping for air and snoring is seen when the airway opens during a resulting arousal from sleep. Oxygen desaturation can be severe as in this case. From top to bottom: EEG channels 1–4. EOG channels 5–6. Chin EMG channel 7. Tibialis anterior EMG channel 8. ECG channel 9. Snoring microphone channel 10. Thermistor and pressure transducer airflow channels 11–12. Chest and abdominal respiratory effort channels 13–14. Oxygen saturation channel 15

Fig. 14.3
Polysomnographic example of central sleep apnea . These events are characterized by complete cessation of airflow due to lack of respiratory efforts. Waves forms noted in the respiratory effort channels are due to sensor detecting heart pulsations. From top to bottom: EEG channels 1–4. EOG channels 5–6. Chin EMG channel 7. Tibialis anterior EMG channel 8. ECG channel 9. Snoring microphone channel 10. Thermistor and pressure transducer airflow channels 11–12. Chest and abdominal respiratory effort channels 13–14. Oxygen saturation channel 15

Fig. 14.4
Polysomnographic example of mixed sleep apnea . These events are characterized by complete cessation of airflow and respiratory effort in the first half of the event (central component). Then respiratory efforts start with persistent lack of airflow (obstructive component) in the second half of the event. From top to bottom: EEG channels 1–4. EOG channels 5–6. Chin EMG channel 7. Tibialis anterior EMG channel 8. ECG channel 9. Snoring microphone channel 10. Thermistor and pressure transducer airflow channels 11–12. Chest and abdominal respiratory effort channels 13–14. Oxygen saturation channel 15

Fig. 14.5
Polysomnographic example of obstructive hypopneas . These events are characterized by partial reduction of airflow. Notice the crescendo snoring during the hypopnea with paradoxical chest and abdominal respiratory movements consistent with partial upper airway obstruction that terminates in an arousal and oxygen desaturation. From top to bottom: EEG channels 1–4. EOG channels 5–6. Chin EMG channel 7. Tibialis anterior EMG channel 8. ECG channel 9. Snoring microphone channel 10. Thermistor and pressure transducer airflow channels 11–12. Chest and abdominal respiratory effort channels 13–14. Oxygen saturation channel 15
Obstructive sleep-disordered breathing is by far the most common of all the three forms. Obstructive events are characterized by complete or partial collapse of the upper airway and persistent respiratory efforts usually in a crescendo pattern terminated by an arousal (Fig. 14.2). Snoring is a sign of partial obstruction and occurs during hypopneas or after termination of an apnea (Fig. 14.5), but it is typically absent during an apnea due to complete obstruction of the upper airway. Snoring is also a prominent feature of the upper airway resistance syndrome (UARS). This is a variant of sleep-disordered breathing that has no apneas, hypopneas, or desaturations. The UARS is characterized by episodes of crescendo snoring and increasingly more negative esophageal and pharyngeal pressure due to crescendo respiratory effort that terminate in an arousal (Fig. 14.6) [52]. These episodes are also known as respiratory effort-related arousals. Clinically, the UARS will present with similar symptoms as OSA [53–55].
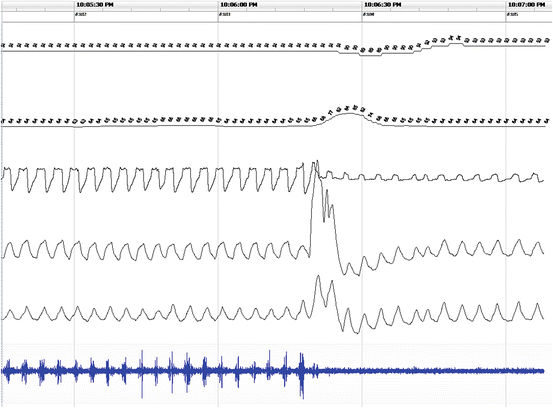

Fig. 14.6
Example of the upper airway resistance during home sleep recording . From top to bottom: SpO2 (channels 1). Heart rate (channel 2). Nasal airflow (channel 3). Thoracic effort (channel 4). Abdominal effort (channel 5). Snoring microphone (channel 6). Notice the airflow limitation or flattening on channel 3 associated with snoring (channel 6) and a minor oxyhemoglobin desaturation of <3 % (channel 1) that resolve after an arousal as measured by a transient rise in heart rate (channel 2)
Central apneas and hypopneas are the consequence of the cessation or partial reduction of the central respiratory drive, thereby resulting in the absence or reduction of respiratory efforts. Typically, snoring is absent, but gasping and some snoring sounds may be heard when respiratory efforts resume after a central apnea or during hyperventilation at the end of a central hypopnea (Fig. 14.3).
Mixed sleep-disordered breathing events have features of both central and obstructive events (Fig. 14.4). Clinically, pure mixed sleep apnea is rare. Mixed apneas and hypopneas generally appear as part of either central or OSA. The type of sleep apnea is determined by the predominant type of event in an individual (i.e., whether more than 50 % of the events are obstructive or central). During such designation, mixed apnea or hypopneas are usually counted as obstructive events since they will respond to CPAP therapy, unlike central sleep apnea.
Except in a few clinical situations, central sleep apnea and Cheyne–Stokes respiration (also known as “periodic breathing” for its waxing and waning airflow pattern (Fig. 14.7)) do not generally occur in the absence of other sleep disorders. Both can be seen transiently during the transition from wake to sleep and in the setting of sleeping at high altitude [30], but these presentations are rarely considered clinically relevant. Central sleep apnea and Cheyne–Stokes respiration disorders can also be seen in the setting of congestive heart failure, use of narcotic pain medications, obesity hypoventilation syndrome, severe central nervous system disease, and idiopathic central sleep apnea [30].
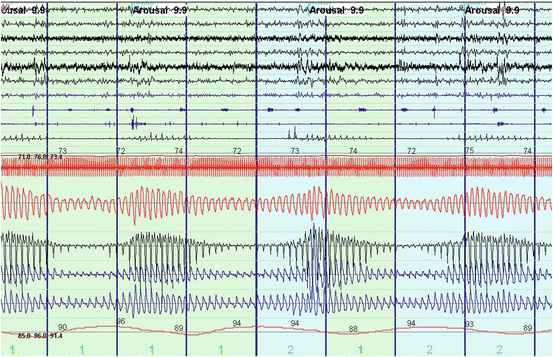

Fig. 14.7
Polysomnogram depicting Cheyne–Stokes ventilation. Notice the waxing and waning airflow pattern (Channels 13–14 from the top). Snoring is absent during the hypopnea but can be seen during the hyperventilation portion of the event (Channel 10). Cheyne–Stokes can result in sleep fragmentation as noted by arousals from sleep (EES channels 1–4) and transient desaturations (Channel 16) that predisposes to periodic breathing through high loop gain physiology
The immediate clinical consequence of central and obstructive sleep-disordered breathing is sleep fragmentation that results in excessive daytime somnolence. Long-term complications of untreated obstructive sleep-disordered breathing syndromes include hypertension, cardiovascular disease, fatigue, depressive mood disorders, and insomnia [24, 25, 56, 57]. In addition, the untreated patient with OSA often complains of a number of psychosocial problems including reduced vigilance, excessive daytime sleepiness, reduced concentration, and deficits of memory and executive function [58], which can result in an increased rate of accidents [59].
Disparities in Obstructive Sleep-Related Breathing Disorders
Snoring
In the 1980s, the self-reported prevalence of snoring was evaluated in a 1222 adult Hispanics living in New Mexico. The prevalence of snoring was greater in men (27.8 %) than in women (15.3 %) and increased with age and obesity for both groups, but the prevalence of snoring leveled off after age 59 for men and women. In this study, snoring was associated with myocardial infarction (odds ratio 1.8; 95 % CI 0.9, 3.6) but not with hypertension after controlling for age, gender, obesity, and smoking. Snorers were also sleepier than nonsnorers, suggesting that snorers may have had a higher prevalence of OSA, which was not directly evaluated in this study [60].
The prevalence of snoring has been evaluated in representative samples from the general US population. In the 2005–2006 National Health and Nutrition Examination Survey, the prevalence of snoring was high at 48 % among 6139 individuals older than 16 years [61]. Results from the National Sleep Foundation Sleep in America 2005 poll of 1506 adults (mean age of 49 years, 51.6 % women) showed that the prevalence of snoring was 59 % and habitual nightly snoring was 40 %. Habitual snoring was more common in men [62]. In the 2007–2008 National Health and Nutrition Examination Survey , sleep symptoms were evaluated by race/ethnicity and socioeconomic position in 4081 non-Hispanic whites, African Americans, Mexican Americans, other-Hispanics/Latinos, and Asians. Non-Hispanic whites represented 72.81 % of the sample, followed by African Americans at 10.29 % and Mexican Americans at 7.55 %. Snoring was more common among males (odds ratio 1.93, 95 % CI 1.66–2.24), other-Hispanics/Latinos (odds ratio 1.37, 95 % CI 1.03–1.83), and those with less than a college education. In this study, African Americans reported more nonrestorative sleep (odds ratio 1.59, 95 % CI 1.25, 2.01) [63].
The Sleep Heart Health Study (1995–2006) is the largest prospective cohort study to assess sleep-disordered breathing and OSA as risk factors for major cardiovascular events, including myocardial infarction and stroke [64]. It evaluated the variations in symptoms of sleep-disordered breathing in 13,194 men and women 40 years of age and older in five major racial/ethnic groups. Snoring was more frequently reported by Hispanic men (odds ratio 2.30, 95 % CI 1.43, 3.69), Hispanic women (odds ratio 2.25, 95 % CI 1.48, 3.42), and Black women (odds ratio 1.55, 95 % CI 1.13, 2.13), compared with the white, American Indian, and Asian Pacific Islander counterparts after adjusting for age, presence of a bed partner, and body mass index [65]. The overall prevalence of self-reported snoring in whites, Hispanics, and African Americans participating in the Sleep Heart Health Study was 34 %. However, the prevalence of self-reported snoring was higher in Hispanics (41 %) compared to whites (34 %), and African Americans (30 %) (p < 0.05) [66].
The Northern Manhattan Study examined a cohort of 1605 older adults (mean age 65 ± 8 years) that included predominantly Hispanics (61 %) but also whites (20 %) and African Americans (19 %). The investigators found the overall prevalence of self-reported habitual snoring was 29 %, but it was much higher in Hispanics (84 %) compared to both African Americans (9 %) and whites (7 %) [67]. In a related study focused on the more elderly participants (mean age 75 ± 9 years, 37 % men), Hispanics more frequently reported habitual snoring (odds ratio 2.8, 95 % CI 1.7–4.5) compared to African Americans and whites. They were also more likely to report long sleep (≥9 h, odds ratio 1.8, 95 % CI 1.1–3.1). There were no differences in sleep complaints between African Americans and whites [68].
The Tucson Children’s Assessment of Sleep Apnea study (TuCASA) examined 1494 questionnaires for children 4–11 years old living in Tucson, Arizona. Parents of male Hispanic children were more likely to report that their boys snore frequently (11.4 % vs. 7.4 %, respectively; p < 0.02) and were more likely to report excessive daytime sleepiness (9.6 % vs. 5.8 %, respectively; p < 0.01) than their white counterparts. There was no difference between Hispanic or white girls with regard to snoring or excessive daytime sleepiness [69]. In children of age 2–6 years attending well-child care visits, the overall prevalence of reported snoring was 13.9 % (95 % CI 10.2, 17.5). However, snoring was found in 18.7 % (95 % CI 13.2, 25.7) of African American children (n = 150), 17.5 % (95 % CI 8.6, 32.4) of Hispanic children (n = 40), and 8.3 % (95 % CI 4.7, 14.4) of white children (n = 132) (p = 0.031). The odds ratio of snoring was 2.5 (95 % CI 1.2, 5.5) for African American children and 2.3 (95 % CI 0.8, 6.4) for Hispanic children as compared to their white counterparts [70].
In summary, it appears that the prevalence of habitual snoring in the general adult US population is high, ranging from 27 to 48 %, and as high as 84 % in the elderly, with the higher estimates coming from studies in more recent years [60–62, 67]. Snoring in general is more prevalent in men than in women and increases with age, but its prevalence plateaus after age 59 [60, 62, 63]. In all the studies that have evaluated snoring and ethnicity, the findings are consistent, showing that habitual snoring is more prevalent in Hispanic men and women, in African American women [63, 65–68], and in Hispanic male children [69] and African American children [70] as compared to their white counterparts.
These studies are limited by the self-reported nature of the snoring data, which can hide significant biases. These studies also do not explain why men, Hispanics, and African Americans snore more frequently than other population groups. Clearly more investigation is needed as to the epidemiology of snoring in the US population.
Upper Airway Resistance Syndrome
The diagnosis of the upper airway resistance syndrome (UARS) is difficult to make using the standard polysomnographic montage, especially when a thermistor is used to measure airflow. Guilleminault used an esophageal manometer to confirm the increasingly negative intrathoracic pressure resulting in an arousal, and this technique is currently the gold standard to make the diagnosis of UARS [53, 54]. Others have used a combination of crescendo snoring associated with increased respiratory effort in the setting of sleep fragmentation to make the diagnosis of UARS [71]. With the advent of the nasal cannula pressure transducers to measure airflow, inspiratory airflow limitation can be determined and used as a measure of increased upper airway resistance to aid in UARS diagnosis [72, 73]. However, noninvasive methods for determining UARS are not yet well validated [54, 74]. The lack of diagnostic consensus has hampered research of UARS in the general population.
In clinical practice, UARS is rarely seen in isolation from OSA [75]. There are very few studies that have addressed the epidemiology of UARS, and all have focused on very specific populations. Kristo et al. reported on 527 patients who underwent evaluation for excessive somnolence in a US military sleep disorders center in 2000 using esophageal manometry during polysomnography. OSA was diagnosed in 72.6 % of these patients as compared to 8.4 % who were found to have pure UARS [76]. In a population of 41 morbidly obese patients (mean BMI 47 kg/m2, 83 % women) referred for bariatric surgery, the prevalence of UARS based on polysomnographic criteria was 17 % as compared to 71 % who were diagnosed with OSA [71].
Stoohs et al. performed a retrospective evaluation of the characteristics of 2753 patients with sleep-disordered breathing seen in two sleep clinics in Germany from 1996 to 2006. They compared patients with primary snoring (n = 157), UARS (n = 424), OSA without sleepiness (n = 562), and OSA with sleepiness (n = 1610). Patients with UARS were significantly younger (45.3 ± 12.3 years) than those with primary snoring (48.7 ± 11.8 years), OSA without sleepiness (53.0 ± 12.3 years), and OSA with sleepiness (OSAS ) (51.5 ± 11.7 years) (p < 0.02). Patients with primary snoring and UARS had a lower body mass index than those with OSA and OSAS (p < 0.001). Also of note, patients with UARS were more likely to be women [77]. No data currently exist examining racial/ethnic or socioeconomic disparities in UARS prevalence.
In summary, the prevalence of UARS in the general population is not known. The current estimates range from 8.4 to 17 % [74, 76], but these figures are based on small research populations with widely different demographic characteristics. However, those with UARS tend to be younger, less overweight, and more frequently female than patients with OSA [71, 77].
The UARS literature is limited by the retrospective nature of the available evidence, the dearth of research in the epidemiology of UARS, the differences in diagnostic methodology used in various studies, and the lack of consensus on UARS as an independent syndrome. Also, the literature has not adequately addressed risk factors related to the prevalence of UARS. Clearly more research is needed to better understand UARS with respect to its epidemiology and potential health disparities.
Obstructive Sleep Apnea
Among all sleep-related breathing disorders , OSA has been the most extensively studied due to its high prevalence and clinical relevance to cardiovascular disease [78]. Epidemiological studies and experimental work in animals support the assertion that OSA, if left untreated, results in activation of the sympathetic nervous system and eventually systemic hypertension [79–81]. Untreated OSA is also strongly associated with other disease states, including coronary artery disease, congestive heart failure and stroke [78, 82], nocturnal arrhythmias [83], diabetes type 2 [84, 85], obesity [86], inflammation [87], hypercogulable state [88], the metabolic syndrome [89], and increased mortality [90]. Since its initial polysomnographic description in 1965 [22], the prevalence of OSA in the US has been increasing in close association with the obesity epidemic [86, 91].
In 1993, Young and colleagues first reported the prevalence of OSA in a random sample of 602 men and women recruited from the general working population ages 30–60 years (Wisconsin Cohort). Based on an AHI ≥ 5/h, the overall prevalence of OSA was 21.3 %. The prevalence of OSA was 24 % for men and 9 % for women. The prevalence of OSA syndrome (OSA plus Epworth Sleepiness Scale score [ESS] > 10) was 4 % for men and 2 % for women [37]. Since then, the prevalence of OSA has been consistently reported as 2:1 to 3:1 male predominance in the premenopausal years and a nearly 1:1 ratio after menopause [92–94]. Peppard et al. reevaluated the Wisconsin Cohort sample from 1988 to 1994 and 2007 to 2010 and adjusted for age, sex, and BMI using sampling weights provided by the respective US National Health and Nutrition Examination Survey (NHANES) for subjects 30–70 years old. The sample consisted of 1520 study participants (96 % non-Hispanic white) who were assessed for sleep-disordered breathing between 1988 and 2011. Women made up 45 % of the sample. They estimated an overall prevalence of OSA (AHI ≥ 5/h) 20 years later at 26 % (95 % CI 24,28). The overall prevalence of OSA syndrome (AHI ≥ 5/h and ESS >10) was not reported. However, the prevalence of OSA (Table 14.1) and OSA syndrome (Table 14.2) was greater for men and women in 2007–2010 as compared to 1988–1994 [91].
Table 14.1
Wisconsin Cohort study
Gender | 1988–1994 data | 2007–2013 data | ||
|---|---|---|---|---|
% | 95 % CI | % | 95 % CI | |
Men | 26.4 | 23.9, 28.9 | 33.9 | 30.8, 37.0 |
Women | 13.2 | 11.4, 15.3 | 17.4 | 15.2, 20.0 |
Table 14.2
Wisconsin Cohort study
Gender | 1988–1994 data | 2007–2013 data | ||
|---|---|---|---|---|
% | 95 % CI | % | 95 % CI | |
Men | 10.8 | 9.0, 12.6 | 14.3 | 12.0, 16.4 |
Women | 3.8 | 2.9, 4.9 | 5.0 | 3.9, 6.3 |
Aging is a major risk factor for the development of OSA and increased prevalence of OSA is found in men and women with increasing age. Ancoli-Israel and colleagues reported that 62 % of community-dwelling older adults (>65 years of age) had significant OSA (AHI ≥ 10/h) based on unattended cardiorespiratory home sleep recordings [95]. The Sleep Heart Health Study also showed the average prevalence of OSA (AHI ≥ 15/h) increased stepwise until approximately age 60, after which it leveled off at a prevalence of about 20 % in a community-dwelling population of 5615 men and women ages 40–98 years [92]. This is very similar to the plateau effect after age 59 seen in the prevalence of snoring described by Schmidt-Nowara et al. [60].
Comparison of the prevalence of OSA by race or ethnicity has been directly evaluated only in a few studies. In the 1990s, Kripke et al. performed overnight oximetry in 190 women and 165 men (age 40–64 years) in southern California, where there is a high proportion of Hispanics of Mexican descent. Based on these data, the authors estimated that 16.3 % of Hispanics had ≥20 episodes of transient oxyhemoglobin desaturation (≥4 %) per hour of sleep, akin to having moderate OSA, compared to only 4.9 % of non-Hispanic Whites [96]. The Hispanic Community Health Study/Study of Latinos (2008–2013) is a multicenter community-based cohort study that evaluated the prevalence of risk factors for sleep disorders and as well as for heart, lung, blood, kidney, liver, endocrine, and cognitive disorders [97]. This study performed unattended home sleep recordings and recently reported the prevalence of OSA for 14,400 participants. The age-adjusted prevalence of OSA for AHI ≥ 5/h, ≥15/h, and ≥30/h was 25.8 %, 9.8 %, and 3.9 %, respectively [41]. Consistent with prior studies, OSA was associated with male sex, obesity, and older age and the prevalence for OSA (AHI ≥ 5/h) was similar to the most recent prevalence estimates in whites [91]. Ancoli-Israel et al. evaluated the prevalence of OSA in elderly African Americans (n = 54) and whites (n = 346) older than 65 years of age. African-Americans had significantly greater AHI than whites (72.1/h vs. 43.3/h; p = 0.014), and there were more African Americans (17 %) with severe OSA (AHI ≥ 30/h) than whites (8 %) (p = 0.034; relative risk = 2.13; 95 % CI 15–19 %). This association remained significant after controlling for age, gender, and body mass index [98]. Redline et al. evaluated 225 African Americans and 622 whites using a cardiorespiratory home sleep recordings and reported that African Americans ≤25 years old had higher AHI and higher prevalence of increased apneic activity (odds ratio 1.88, 95 % CI 1.03–3.52) after adjusting for obesity, sex, proband sampling, and familial clustering. The authors concluded that young African Americans may be at increased risk for sleep apnea [99]. There are no studies directly comparing the prevalence of OSA between Asians and whites. However, in middle-aged Asian men and women the prevalence of symptomatic OSA diagnosed by polysomnography (AHI ≥ 5/h plus ESS >10) has been reported as similar to that in whites (4.1–7.5 % for men and 2.1–3.2 % for women) [37, 100–103].
In summary, OSA is strongly associated with male gender [37, 92–94], obesity [86], and increasing age [92, 96], but there appears to be a plateau effect on prevalence after age 60 [92]. There are very few studies that have directly examined differences in the prevalence of OSA by race or ethnicity, and those only compared African Americans with whites. However, from the available literature, it appears that both younger and older African Americans are more likely to have OSA compared to whites [98, 99]. In Hispanics , the prevalence of OSA was estimated to be higher than that of whites based on overnight oximetry [96]. However, based on unattended home sleep studies the prevalence of OSA in US Hispanics was similar to that of whites [41]. The prevalence of symptomatic OSA in Asians has been reported similar to that of whites [37, 100–103]. There is a temporal trend of increasing OSA prevalence over time. The OSA prevalence in the general population that was described by Young et al. in 1993 (4 % for men and 2 % for women) is still quite commonly cited by sleep researchers and clinicians today [37]. However, with the obesity epidemic [86, 91] and the aging of the population, it is highly likely that the prevalence of OSA in the US has increased. Our most recent estimates of OSA prevalence come from the mostly white population of the Wisconsin Cohort Study in 2013 (Tables 14.1 and 14.2) and the direct measurements in the Hispanics Community Health Study/Study of Latinos in 2014, showing a definite increase in the prevalence of OSA over time [41, 91, 98].
Methodological differences make it challenging to compare earlier and later studies of OSA prevalence because of changes in measurement of hypopneas. Earlier sleep recordings utilized primarily the thermistor to evaluated airflow [37], which is notable for underestimating hypopneas, while later studies have used primarily the nasal cannula pressure transducer which is a better detector of hypopneas and airflow limitation [104, 105]. Also, earlier studies used “discernible” reductions in flow to identify hypopneas [37], while later studies have used more precise airflow decrements of 30 and 50 % detected by nasal cannula pressure transducers [51]. Moreover, earlier studies generally utilized a ≥4 % oxyhemoglobin desaturation to designate hypopneas [37], while later studies have also used the 2007 American Academy of Sleep Medicine alternate definitions of hypopneas that included a ≥3 % desaturation and/or a resultant arousal [51]. In all, the prevalence of OSA in the US population is probably significantly greater now than that reported in 1993 due to a true increase in OSA severity caused by the obesity epidemic and the aging of the population, as well by more accurate detection of hypopneas due to advances in technology.
Disparities in the Clinical Presentation of OSA
The clinical presentation of OSA differs significantly by sex. In a retrospective study, Shepertycky et al. evaluated 130 men and 130 women with OSA who were matched for age, BMI, AHI, and the ESS score. Both men and women presented similarly with respect to snoring and excessive daytime sleepiness. However, women were more likely to have a main presenting complaint of insomnia (odds ratio 4.20; 95 % CI 1.54–14.26). Women were also more likely to endorse a history of depression (odds ratio 4.60; 95 % CI 1.71–15.49) and hypothyroidism (odds ratio 5.60; 95 % CI 2.14–18.57). Compared to their male counterparts, women were less likely to present with witnessed apnea and consumed less caffeine per day [106].
In the Sleep Heart Health Study, the severity of sleepiness increased with increasing OSA severity and with increasing frequency of snoring for both men and women, regardless of age or BMI [107, 108]. In a study that evaluated complaints of insomnia in men and women with OSA, women reported sleep onset insomnia more frequently than men (62 % vs. 53 %, p = 0.03) as well as psychophysiologic insomnia (53 % vs. 45 %, p = 0.03) more frequently than men [109].
In a more recent study of 384 African American and white adults that were evaluated with the Berlin Questionnaire [110], women with OSA reported fatigue more frequently than men with OSA (75 % vs. 46 %, p < 0.001). In multivariate analysis that adjusted for potential confounders, men with OSA were sleepier than women, and African American men were significantly sleepier than white men (Average ESS 12.8 ± 5.2 for African American men as compared to 10.6 ± 5.3 for white men, p = 0.05) [111]. In a retrospective study of 383 women and 661 men diagnosed with OSA, women were older and had a greater BMI and waist-to-hip ratio than men at the time of diagnosis. OSA severity based on respiratory disturbance index (RDI) was higher in men than women (41.2 ± 27.9 vs. 30.0 ± 26.7, p < 0.001) despite a greater BMI in women [112]. In a population of 300 OSA patients (AHI > 10/h), the same investigative team again noted that women with OSA were older, had greater BMI, and had lower AHI at diagnosis compared to men [109].
Simpson et al. evaluated the relevance of fat distribution in men and women with OSA by measuring fat with dual-energy X-ray absorptiometry. The proportion of obese men and women did not differ (68 %, BMI > 30), and in evaluation of the upper airway, there was no difference in the proportion with a Mallampati score of III or IV by sex. In women, the combination of percentage of fat in the neck region and body mass index together explained 33 % of the AHI variance. In men, percentage of fat in the abdominal region and neck-to-waist ratio together accounted for 37 % of the AHI variance [113].
There are few studies directly evaluating ethnic/racial differences with respect to the clinical presentation of OSA. In a study by Scharf et al., African American OSA patients were younger at the time of diagnosis (44.9 ± 14.1 vs. 49.2 ± 14.5 years; P = 0.022) and had greater BMI than whites (39.7 ± 10.7 vs. 33.4 ± 9.2 kg/m2; p < 0.0001). However, there was no difference in the severity of OSA between whites and African Americans after controlling for BMI and median household income [114]. Subramanian et al. evaluated the gender and ethnic differences in the prevalence of insomnia in 300 patients with OSA (AHI > 10/h) that included white, African American, and Hispanic men and women. White women were more likely to complain of sleep maintenance insomnia and Hispanic women were more likely to complain of psychophysiologic insomnia [109].
In summary, there are significant gender and racial/ethnic differences in the clinical presentation of OSA that could have diagnostic and therapeutic implications. Like men, women with OSA present with snoring and excessive daytime somnolence [106–108]. However, women tend to be older, have a greater BMI [94, 109, 112], and demonstrate greater importance of neck fat in upper airway patency [113]. Women also present more frequently with a chief complaint of insomnia, fatigue, or depression, which can lead to a misdiagnosis or delayed diagnosis of OSA [109, 111]. Less data exist regarding the racial/ethnic differences in the clinical presentation of OSA. However, African Americans tend to be younger and more overweight at the time of diagnosis than whites. There does not appear to be a racial/ethnic difference in OSA severity at presentation [114]. More research is needed, especially comparing racial/ethnic differences in the clinical presentation of sleep-disordered breathing.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


