Fig. 5.1
Underdiagnosis of COPD in the absence of routine spirometry. Reproduced with permission from Insights for Improvement: Advancing COPD Care Through Quality Measurement. Copyright © 2009 by the National Committee for Quality Assurance (NCQA). To access a copy of this publication, visit http://www.ncqa.org
Even among patients who do receive a diagnosis of COPD , spirometry is underutilized. In a 2007 review of over 5000 patients newly diagnosed with COPD, just 32 % had any PFTs prior to disease diagnosis [4]. Similarly, a review of around 200,000 Veteran Affairs patients newly diagnosed with COPD found the frequency of spirometry testing was around 34 % [5]. Both studies found the frequency of spirometry testing lower among older patients. Additionally, several studies have established that the burden of asthma and respiratory disease is not uniform across all patient groups. Ethnic minorities and those with lower socioeconomic status (SES) have increased morbidity and mortality related to asthma, with lack of access to healthcare, including PFTs, implicated [6–13].
The lack of routine PFTs in these settings has a number of potential consequences for patient care. Limited use of PFTs in the initial evaluation of patients with possible COPD has been shown to increase the risk of underdiagnosis of COPD. Confirmation of the diagnosis of asthma/COPD with PFTs results in better overall care, giving providers greater confidence in prescribing medications appropriately for moderate to severe COPD, such as anticholinergic inhaler therapy and long-acting beta agonists [14]. Additionally, there is a risk of misdiagnosis or “overdiagnosis ,” with patients being exposed to treatments for conditions they don’t have, as well as delayed or missed diagnosis of other serious disorders. Misdiagnosis can also lead to patients being “labeled” with chronic medical conditions, with resulting psychosocial stigmata, as well as increased premiums for health and life insurance. When COPD or asthma is misdiagnosed, often due to lack of spirometry, prescription of inhaled bronchodilator medications can be ineffective and wasteful. This is particularly true when patients experience an apparent response to therapy because of spontaneous resolution of self-limited conditions such as viral respiratory tract infections. This can result in undesirable medication side effects, as well as financial burden from having to purchase inhalers or nebulizers. Many inhalers have a monthly cost of $100–$300, so this financial burden may be considerable.
Many factors may contribute to underutilization of spirometry in primary care settings, , but exact reasons for underutilization have not been adequately explored. Many laboratories have substantial wait times prior to pulmonary function testing being available. Long waiting times (as long as several weeks) may be a sufficient disincentive to prevent some otherwise appropriate testing. In our pulmonary function laboratory, we operate with the assumption that “unmet demand goes away” and manage test availability with a targeted maximum wait time of 2 days and immediate accommodation of patients who show up requesting immediate testing.
Perhaps the greatest contributing factor to lack of test availability is lack of the most expensive component of the testing system: well-trained staff who can coach patients to perform acceptable and repeatable PFTs. Comprehensive technician training and a program of quality assurance and feedback have been shown to improve the quality of spirometry results [15]. The American Thoracic Society (ATS) and the European Respiratory Society (ERS ) have published standardization guidelines for spirometry [16, 17]. In surveys of general practitioners, lack of confidence in the quality of the spirometry measure was felt to be a major limitation to widespread utilization of PFTs in primary care practice [18]. Prior studies noted that spirometry performed in family practice settings without technician training frequently does not satisfy ATS/ERS criteria for acceptability and repeatability. However, following relatively simple training, the proportion of maneuvers achieving quality standards increased substantially, highlighting the importance of effective training and quality assurance programs to ensure successful spirometry in primary care practice [19]. Crucially, several studies have demonstrated that when spirometry is performed in primary care practices by healthcare professionals trained to appropriately coach patients through spirometry maneuvers, the quality of spirometry results was comparable to those performed in a specialist-run pulmonary function laboratory [20].
As noted earlier, the utilization of PFTs in newly diagnosed COPD cases is lower among older patients [4, 5]. Part of this age discrepancy may be out of concern for poor test quality in older adults . In the SARA study , spirometry reproducibility and acceptability was assessed for older adults (>65 years) undergoing PFTs for the diagnosis of asthma or COPD. Investigators found that testing in older adults typically required more time and more attempts. However, if this was done with rigorous quality control measures implemented, good spirometry test quality was achievable with result quality comparable to that among younger patients. Cognitive impairment, a shorter 6-min walk distance, and lower educational level were all found to be independent predictors for a poorer acceptability rate [21].
Spirometry certification programs exist both nationally and internationally to provide the necessary training. In the United States, certification was established through the Cotton Dust Standard [29 CFR 1910.1043] and is coordinated by the National Institute for Occupational Safety and Health (NIOSH ). Initial certification requires 15 h of training. A 1-day recertification program is required every 5 years. In addition to time and money, another potential roadblock to certification is not having geographical proximity to one of the NIOSH-approved training centers [22]. In primary care clinics where adequate technician training limits the availability of quality PFTs, a possible alternative is spirometry performed locally within the clinic, but coordinated and coached online by certified technicians working at a separate pulmonary function laboratory. This concept has been shown to provide an adequate alternative to conventional spirometry in primary care centers [23]. Once staff members have been appropriately trained to perform spirometry or other tests, ongoing staff management is required to provide test availability when needed. In the primary care setting , this includes juggling of other duties for busy staff who may be nurses, respiratory therapists, phlebotomists, or other laboratory or clinical personnel. Most have other tasks that must be prioritized.
Equipment availability, by contrast, is probably rarely a legitimate limitation to availability of PFTs in the United States and other developed countries. Although pulmonary function equipment capable of complete PFTs (including lung volumes and diffusing capacity in addition to spirometry) typically cost more than $30,000, a simple office spirometer can be purchased for under $1000, and a more comprehensive spirometry system can be purchased for less than $5000 complete with computer and printer. Medicare reimbursement for spirometry with bronchodilator is over $70 per test, though Medicaid reimbursement is less. The test requires 20–30 min of staff time and the only disposable cost is for a filter/mouthpiece, which typically costs $1–5. Reimbursement for healthcare services in the United States typically underpays for cognitive services but overpays for procedural services, so the underutilization of spirometry seems curious and illogical. As stated earlier, we believe lack of timely test availability is a major unrecognized contributing factor.
As discussed elsewhere in this text, asthma has increased disease prevalence among low-income and inner-city communities, those with lower educational attainment (high school or less), women, and African-Americans [13]. Over time, there has been a widening of the racial differences in asthma severity , with studies suggesting African-Americans may be more likely to have asthma hospitalizations, greater severity of disease, and higher asthma-related mortality [8, 10–12]. Cigarette smoking remains a major modifiable risk factor for developing respiratory diseases, and the burden of cigarette smoking is similarly disproportionate—greater in those living below the poverty level, with lower educational levels, and within certain ethnicities (particularly Native Americans) [24, 25]. The prevalence of cigarette smoking has declined over time, thanks to concerted public health campaigns, increased taxation on cigarettes, prohibition of indoor cigarette smoking, antitobacco mass media campaigns, and barrier-free access to smoking cessation aids [24]. These public health efforts need to continue and should be focused on communities and groups in which prevalence of cigarette smoking remains high. Inner-city communities also have increased exposures to airborne particulate matter and other vehicle emissions [26, 27]. These exposures have been linked to declines in lung function in the general population [27, 28], which are likely even greater for the elderly and patients with chronic respiratory conditions [29]. Other potentially significant exposures seen more commonly among inner-city residents include inhaled drugs such as crack cocaine and greater exposure to certain antigens such as from cockroaches and dogs [30–32].
Inner-city and poorer communities also have lower doctor-to-patient ratios, resulting in less time per clinic visit, less continuity of care, greater proportion of care in emergency room and urgent care settings, as well as longer waiting times for routine appointments [33, 34]. Quality of asthma care has been shown to be lower for inner-city patients with asthma, particularly within ethnic minorities and those living in poorer neighborhoods [6, 7, 9, 12]. In these time-pressured settings, often in urgent care centers without established patient–provider relationships, diagnostic accuracy including evaluation with spirometry may be neglected in favor of “quick fixes” such as empiric antibiotics, rescue inhalers, and oral corticosteroids. Patients may have lower rates of insurance coverage and indicated diagnostic testing such as spirometry may be skipped because of patients’ inability to pay [34].
Another barrier to access may be lack of transportation . In the inner-city setting, testing facilities are likely to be close—within a few miles of a patient. However, in the absence of affordable, accessible, and safe mass transit systems, getting to clinics may be time or cost prohibitive. In the rural setting , including Native American reservations, the opposite problem exists, where distances may be great. Consequently, patients require not only vehicles, but also money for gasoline and the ability to take time off from work. Again, this can be time and cost-prohibitive. The ultimate result is less access to PFTs, and a greater proportion of patients with underdiagnosed, misdiagnosed, or mismanaged pulmonary conditions, including not only asthma and COPD, but also other less common conditions which are frequently misdiagnosed as asthma or COPD until more thoughtful evaluation is pursued.
Language barriers and Pulmonary Function Testing
Patients with limited English proficiency face additional barriers to accessing high-quality PFTs in the United States. It has been well established that ensuring adequate communication in the healthcare setting is critical in providing safe and efficacious care for those with limited English proficiency [35]. Language barriers in the United States and around the globe can lead to suboptimal healthcare delivery and poor health outcomes [36]. Federal requirements in the United States related to culturally and linguistically appropriate services have been crucial in helping to change how medical interpretation is viewed. The Office of Minority Health developed “National Standards on Culturally and Linguistically Appropriate Services” to help hospitals ensure quality care for diverse patient populations [37]. Despite these standards, clinics and hospitals often still utilize family members and friends to serve as interprete rs. Additionally, the use of interpreters accessible by telephone or other communications media has risen drastically, especially in areas with limited availability of in-person services. While telephone interpreters provide a much needed service, they lack the ability to provide the nuances of face-to-face interactions that can be critical in many medical interactions.
The diversity of the United States population continues to increase. According to data collected from NHANES from 1999 to 2004, nearly 16 % of the sampled individuals spoke a language other than English, which was most commonly Spanish (96 %, though Mexican-Americans were oversampled) with the remaining individuals (4 %) speaking 30 different languages. Language interpreters must work “on the spot” and convey spoken words from one language to another. A common misconception is that individuals (including clinic staff and patient families) that are bilingual can serve as interpreters with no difficulty; however, those with no formal training are more likely to add or omit information in an exchange between examiner and patient. In addition, individuals who are unfamiliar with using language interpreters often do not speak directly to the patient, which can alienate that individual. They also tend to speak in long sentences, can unintentionally patronize or infantilize adults with limited English abilities, and often raise their voice, although the patient is not hearing impaired [38].
Language barriers are not insurmountable. At Mayo Clinic Rochester, interpretation services for 17 languages are provided by in-person interpreters available daily, including American Sign Language, Arabic, Bosnian/Croatian/Serbian, Cambodian, Chinese Mandarin, Dinka, French, Italian, Lao, Somali, Spanish, Swahili, Taiwanese, and Vietnamese. With advanced notice interpretation services are available for Greek, Hebrew, Japanese, Korean, and Russian speakers. A “Language Line” telephone interpreter service is available for many additional languages and dialects. Olmsted County, in which Mayo Clinic Rochester is located, is one of the fastest growing counties in Minnesota, with Rochester being the fastest growing metropolitan area in the state. According to the 2006 Census, the resident population of Olmsted County is 137,521, with 70 % of the population living in Rochester. Minorities (races other than White or Hispanic-Latino ethnicity ) make up 11 % of the county’s population [39]. Enrollment in the Rochester Public Schools shows that 20.9 % of students are classified as minority. Diversity is further reflected in the fact that K-12 students speak more than 53 languages in their homes [39]. Despite the vast resources available at such tertiary medical centers to provide interpreters during patient visits and other testing, there remain instances when family and friends are relied upon to provide interpretation. Sadly, not all medical centers have such resources, and at times there is no access to even basic translation services.
The importance of language and communication is evident in PFTs, where the technician must work closely with the patient to ensure proper technique. The importance of the use of medical translator s in the pulmonary function lab is exemplified by a 31-year-old Somali-speaking woman who was referred to Mayo Clinic for further evaluation of abnormal PFTs. The outside pulmonary testing was performed for nonspecific cough, tightness in throat, and episodic shortness of breath following an upper respiratory tract infection (URI). There was no evidence of wheezing or other pulmonary abnormality on physical exam, with the only pertinent physical finding being morbid obesity.
Spirometry performed without an interpreter at an external site is shown in the left panel of Fig. 5.2, demonstrating evidence of severe obstruction (FEV1 0.89 L, 31 % predicted; FVC 2.10 L, 62 % predicted; FEV1/FVC 42.4 %). She was also reported to have a severe reduction in DLCO (8.0 mL/min/mmHg, 30 % predicted) and a small improvement after bronchodilator. The outside provider ordered a CT scan of the chest, which was negative, and referred the patient for pulmonary, otolaryngology (ENT), and gastroenterology consultations. Repeat spirometry, performed with a Somali interpreter , is shown in the right panel of Fig. 5.2. The repeat test shows borderline restriction, likely secondary to obesity, with no evidence of airflow obstruction (FEV1 2.27 L, 79 % predicted; FVC 2.55 L, 75 % predicted; FEV1/FVC 89.0 %) or bronchodilator response and normal DLCO (24.2 mL/min/mmHg, 99 % predicted). This example demonstrates how the services of an interpreter drastically improved the patient’s ability to perform PFTs. Without the additional data provided by the second round of pulmonary testing, the patient might have undergone additional unnecessary testing, and she might have been erroneously labeled with severe lung disease and treated with expensive and potentially harmful medications. The use of interpreters for PFTs for non-native-English speakers has not been directly studied; however, experience shows that coaching a patient in their native language provides the best results.
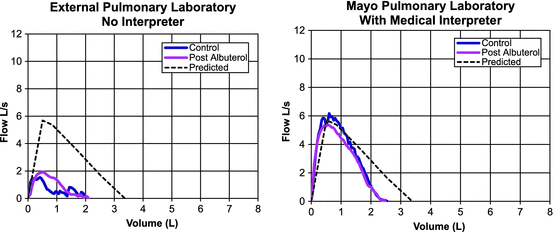

Fig. 5.2
Comparison of spirometry performed with and without a medical interpreter. Left panel shows spirometry performed with no interpreter. Severe obstruction is noted with a severe reduction in DLCO. Some improvement with bronchodilator noted. Right panel shows near normalization of the spirometry curve when testing is repeated with assistance of a Somali interpreter (patient’s native language) to coach the patient through the maneuvers
Description of Respiratory Symptoms: Cultural Variations among English Speakers
Even among English speakers, cultural variations in language utilization and word selection can create communication barriers . Caucasians and African-Americans may select different words to describe the symptom of dyspnea [40]. Healthcare providers may fail to recognize that their patient’s symptoms are due to respiratory disease resulting in underutilization of PFTs. In a study of African-Americans with asthma in Nashville, Tennessee, participants were broken into focus groups where they discussed their perception of asthma symptoms and severity. Common symptom descriptions included breathing problems, chest tightness or pain, wheezing, sweating, and dizziness. Overall, it was found that the study participants denied feeling that they were unable to “get air in” [41]. In a follow-up study, both African-American and Caucasian study participants were asked to complete a questionnaire reporting asthma symptoms, and it was found that African-Americans use descriptive terms to report their symptoms that differ from Caucasians [42]. Nocturnal awakenings, dyspnea, chest pain, throat pain, and fatigue were all descriptors used to report asthma symptoms that were used more frequently by Caucasians than African-Americans [42].
In another study, differences between word descriptors of dyspnea were assessed among four different groups, including African-Americans, Hispanic-Americans, Asian-Pacific Islanders, and Caucasians [40]. Caucasian subjects used primarily lower airway ethnic word descriptors (EWDs) , such as chest heavy, wheezing, deep breathing, out of air, and hurts to breathe. There were several distinct upper airway descriptors used by Asian-Pacific Islanders and African-Americans, including itchy throat, itchy, itchy at back of throat, tight throat, and cough. Hispanic-Americans used both upper and lower airway word descriptors to describe their symptoms. This study showed different ethnic groups used different terms to describe the same disease process and symptoms. The use of upper airway terminology goes against what most healthcare providers have been taught to associate with breathlessness [40]. This indicates that minority patients may use words to describe an acute asthma flare that some healthcare providers do not associate with the disease.
Health Disparities and Interpretation of PFTs
Reference Values in Pulmonary Function Testing
An important component of interpretation of PFTs is comparing the measured result to a reference standard or predicted value. This is usually reported as a “percent predicted” for each measurement. Reference values vary with sex, age, height, and ethnicity. For example, a healthy 60-year-old white woman who is 165 cm (5′5″) tall is expected to have an FEV1 value of 2.65 L whereas a healthy 25-year-old white man who is 188 cm (6′2″) is expected to have an FEV1 value of 5.10 L [43]. PFT reports include the value of each lung measurement as well as the predicted normal value and the percent of the predicted value. If the 60-year-old woman had a measured FEV1 of 2.12 L, she would be reported to have FEV1 of 80 % percent predicted.
The “percent-predicted” value is used to diagnose disease or quantify the severity of disease. For example, the widely used GOLD criteria (Table 5.1) for chronic obstructive pulmonary disease (COPD) categorize the severity of COPD based on the percent-predicted value for the postbronchodilator FEV1 [44]. Patients with different degrees of severity are often prescribed different medications or therapies.
Stage | FEV1 (%) | Severity |
|---|---|---|
I | >80 | Mild |
II | 50–79 | Moderate |
III | 30–49 | Severe |
IV | <30 | Very severe |
Accurate reference values are extremely important when using PFTs to diagnose disease. Flawed calculation of reference values can lead to underdiagnosis or overdiagnosis of disease. Numerous reference equations have been developed to calculate normal values. The ATS and ERS lung function testing guidelines published in 2005 list over three dozen published spirometry reference equations from around the world [45]. These reference equations are generally derived from large groups of healthy nonsmoking individuals with presumed normal lung function who have volunteered to undergo spirometry testing. No single reference equation is used universally, and the ATS/ERS 2005 guidelines do not recommend a particular reference equation. Rather, they recommend that each pulmonary function laboratory selects reference equations derived from a population that is similar to the patients tested by the laboratory [43].
The most widely used reference equations in the United States were derived from the National Health and Nutrition Examination Survey (NHANES III ) and reported by Hankinson and colleagues in 1999 [43]. NHANES III was a random sample from the U.S. population of 20,627 individuals (including 16,484 adults). The study included an intentional oversampling of African-American and Mexican-American individuals (Fig. 5.3). Participants completed a health survey and collection of health data including standardized spirometry measurements. After excluding individuals with known or suspected lung disease and cigarette smokers, 4634 adults age 17–80 were included in the final dataset used by Hankinson to derive spirometry reference equations. Three separate equation sets were developed including Caucasian Americans, African-Americans, and Mexican-Americans. No equations were developed for Asian Americans because of lack of statistical power for that group.
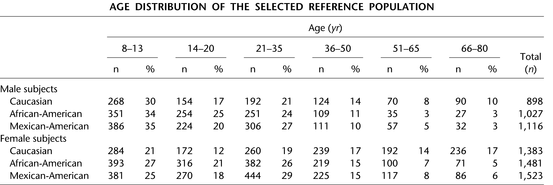

Fig. 5.3
Demographics from NHANES III reference population. Reprinted with permission of the American Thoracic Society. Copyright © 2014 American Thoracic Society. Hankinson JL, Odencrantz JR, and Fedan, KB. Spirometric Reference Values from a Sample of the General U.S. Population. Am J Respir Crit Care Med 1999; 159:179–187. Official Journal of the American Thoracic Society
The Global Lung Initiative (GLI) equations,, published in 2012, are an effort to develop international spirometry reference equations [46]. The GLI authors pooled spirometry data at the individual level from previous studies of healthy asymptomatic nonsmokers, including data from NHANES III [43] and MESA [47]. The result was the largest yet single dataset of spirometry results from asymptomatic nonsmokers, consisting of 74,187 individuals across 26 countries. This dataset was then used to generate reference equations for calculation of percent-predicted normal spirometry values. Unfortunately, despite this great effort, the 2012 version of the GLI equations still are not universal spirometry equations as they did not include enough individuals from some parts of the world including Arab, Asian Indian, Polynesian, sub-Saharan African, and Latin American peoples [46].
“Race Correction” in Pulmonary Function Testing
Differences have been reported in comparing individuals of different ethnicity or race. Reference equations generally use age, gender, and height in calculation of predicted normal values. A few have race- or ethnicity-specific equations but most apply a multiplier or “race correction factor” for members of races or ethnicities other than the predominant group. Current ATS/ERS guidelines (2005) do recommend the use of “race correction” factors for interpretation of PFTs and recommend that self-identification be used to define a subject’s race [45]. The 2005 guidelines state: “The subjects being tested should be asked to identify their own race/ethnic group, and race/ethnic-specific reference equations should be used whenever possible. If such equations are not available or are unsuitable for a particular setting, a race/ethnic adjustment factor based on published data may be used for lung volumes” [45]. This is based on the observation that variations in stature and environmental or socioeconomic factors do not fully explain the observed differences in lung function between racial/ethnic groups. The concept of “race correction” is problematic and controversial [48].
The NHANES III study found a difference in spirometry results by race and ethnicity (Fig. 5.4). The authors observed that the lower FEV1 values obtained from Mexican-Americans can be attributed to shorter heights, but that African-Americans have lower FEV1 values even after adjusting for height. The authors speculate that this may be due to difference in body build, only partly explained by the fact that African-Americans may have a smaller trunk-to-leg ratio than Caucasians, that is, a smaller trunk at any given height, hence smaller lungs. A subsequent analysis of NHANES data suggested that differences in SES and body habitus between Caucasians and African-Americans accounted for only about half of the observed racial difference in FEV1 and FVC [49]. On the basis of these observations, Hankinson reported separate equations for Caucasians, African-Americans, and Mexican-Americans. The GLI authors observed similar findings. Lung function in the GLI dataset was similar between white Europeans and Mexican-Americans after correcting for height, and the GLI authors report a single reference equation (“Caucasian”) for use with individuals from both groups. Among African-Americans, lung function was observed to be lower compared to “Caucasians” even after adjustment for height by a mean difference of 14.7 % for men and 13.8 % for women for FEV1 [46].
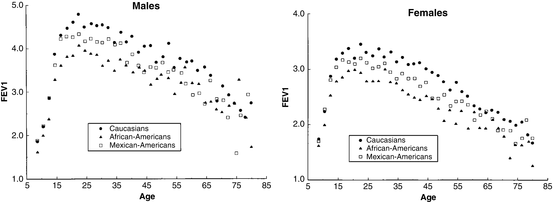

Fig. 5.4
Relationship between race and FEV1 for men and women in asymptomatic nonsmokers from NHANES III reference population. Reprinted with permission of the American Thoracic Society. Copyright © 2014 American Thoracic Society. Hankinson JL, Odencrantz JR, and Fedan, KB. Spirometric Reference Values from a Sample of the General U.S. Population. Am J Respir Crit Care Med 1999; 159:179–187. Official Journal of the American Thoracic Society
The NHANES III survey population did not include enough individuals of Asian ancestry (Eastern or Southern) to assess the ethnic differences in lung function from these groups. Therefore, no separate NHANES equations are available for use in testing patients from these ethnic groups. For Asian-Americans, the ATS/ERS 2005 guidelines recommended using a correction factor of 0.94 applied to reference values obtained from the Caucasian equations [45]. Only two references are cited by the guidelines in support of this correction factor [50, 51]. One study examined 3076 elderly Japanese-Americans (ages 71–90) residing on the island of Oahu, Hawaii [51]. The other cited study was a small study sampling 80 medical students and physicians between age 22 and 33 that included 40 whites and 40 Asian-Americans [50]. The authors found slightly lower lung function measurements in Asian-Americans compared to whites after correcting for age, length of residence in the United States, activity level, baseline characteristics, and anthropometric measurements.
A correction factor of 0.88 was subsequently proposed for Asian-Americans based on results of the 2010 Multi-Ethnic Study of Atherosclerosis (MESA) Lung study [47] which evaluated spirometry in 1068 healthy nonsmokers. The study group was ethnically diverse, including 25 % white, 20 % African-American, 32 % Asian-American, and 23 % Hispanic (including roughly half Mexican and half non-Mexican Americans) individuals. The purpose of the study was to validate the NHANES III reference equations in a large multiethnic adult population. The NHANES III equations performed well when applied to the MESA-Lung patients who identified themselves as whites, African-Americans, and Hispanics. However, the authors observed that the NHANES III equations consistently overestimated lung function in Asian-Americans even when the 0.94 correction factor was used. They therefore proposed that a correction factor of 0.88 applied to the NHANES III equations for Caucasians should be used to achieve the best fit for Asian-Americans. The authors note that the MESA-Lung study population included primarily Asian-Americans of Chinese origin. Therefore, the observed difference in correction factor may be due to differences in lung function between Americans of Japanese and Chinese ancestry. No recommendation was made regarding patients of South Asian ancestry.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


