CLINICAL AND PREVENTIVE CARDIOLOGY
CHAPTER
Hallmarks of Essential and Secondary Hypertension
A number of pathophysiologic factors have been implicated in the development of essential hypertension, making selective mechanistically based antihypertensive therapy in any one patient difficult.1 In a broad sense, increased sympathetic nervous system activity, autonomic imbalance (increased sympathetic tone, abnormally reduced parasympathetic tone), vascular remodeling, arterial stiffness, and endothelial dysfunction contribute to both the development and maintenance of essential hypertension. Increased sympathetic activity may stem from alterations in baroflex and chemoreflex pathways, both peripherally and centrally. The renin–angiotensin system plays a major role in vascular remodeling (alterations in structure, mechanical properties, and function of small arteries) and critical target organ damage (TOD) (myocardial fibrosis, renal injury). In addition, arterial stiffness—a primary contributor to increased vascular resistance, especially with advancing age—results from continued collagen deposition, smooth muscle hypertrophy, and changes in the elastin media fibers. Although intact vascular endothelium is critical to maintaining vascular tone (relaxation and contraction), we now know that multiple insults (decreased nitric oxide synthesis, increased endothelin, estrogen deficiency, high dietary salt intake, diabetes mellitus, tobacco use, and increased homocysteine) can damage vascular endothelium and contribute to important clinical findings. These vascular factors or conditions disrupt normal endothelial function, initiating the cascade of cardiovascular events that results in atherosclerosis, thrombosis, and heart failure.
Renal microvascular disease remains a viable theory as being responsible for the development of hypertension.2 Renal vasoconstriction resulting from the renin–angiotensin–aldosterone system (RAAS) activation, increased sodium reabsorption, and primary microvascular injury may all lead to renal ischemia (particularly in the outer medullary section). Local production of angiotensin-II plus reactive oxygen species at sites of renal injury potentially result in structural alterations and hemodynamic events that cause hypertension.3
Hyperuricemia in humans is associated with renal vasoconstriction, activation of the RAAS, cardiovascular disease (CVD) risk, and hypertension. Theoretically, uric acid stimulates renal afferent arteriopathy and tubular interstitial disease, resulting in hypertension. Renal lesions and hypertension could be prevented or reversed in a rodent model by decreasing uric acid levels coupled with use of angiotensin-converting enzyme (ACE) inhibition, but not hydrochlorothiazide (HCTZ).4 Continued studies leveraging these observations in humans warrant further investigation. Moving forward, medication selection may be directed as much to specific detrimental microvascular effects as to the actual lowering of blood pressure (BP) to target levels.
GENETICS OF HYPERTENSION
Hypertension results from a complex interaction of genetic, environmental, and demographic factors. In patients with essential hypertension, heritability (h2) has been estimated to range from 30% to 50% indicating that a large proportion of variation in BP can be attributed to additive genetic effects. Variation in BP appears to be the result of contribution by several different genes (it is polygenic).5 In addition, there are reported rare cases of simple Mendelian forms of high BP in which a single gene defect may be largely responsible for the hypertensive phenotype.
Improved techniques of genetic analysis (i.e., genetic-wide linkage analysis) have aided in the search for genes that contribute to the development of primary hypertension. Genetic causes of hypertension, though uncommon in the general population, may be more frequent in selective hypertensive populations, particularly patients with resistant hypertension. Genome scans have identified regions of specific human chromosomes that influence BP, which are called the BP quantitative trait loci (QTL) (i.e., chromosome 6.2). Lack of standardization in BP measurement, dichotomization of BP levels for diagnosis of hypertension, and inappropriate selection of cases and controls in clinical studies may result in substantial variation in the hypertension phenotype, which may have contributed to the slow progress in identification of genetic variants associated with BP. Consequently, genetic profiling is not currently beneficial in the diagnostic evaluation of hypertension.
From the clinical perspective, a family with a history of hypertension can be a surrogate marker for undefined, genetically linked risk factors shared by the family. Risk factors such as obesity, dyslipidemia, and insulin resistance are predictive of future hypertension. Having a single first-degree relative with hypertension is only a weak predictor of hypertension, whereas a finding of two or more relatives with hypertension at an early age (before age 55 years) identifies a smaller subset of families who are at much higher risk for the future development of hypertension.6
Wilk et al.7 reported findings to support a link between the quantitative trait age at hypertensive diagnosis and the qualitatively defined early-onset trait in African Americans. Several genes with specific salt interactions have been identified, for example, ones for glucocorticoid remedial hypertension and apparent mineralocorticoid excess.8 In addition, the α-adducin gene is associated with an increased risk of renal tubular absorption of sodium, and angiotensinogen gene polymorphism (A-to-G substitution and methionine-to-threonine amino acid substitution) has been linked to an increase in plasma levels of angiotensinogen.9
Patients with specific gene patterns may respond preferentially to one class of drugs more than another. Patients with the α-adducin gene respond best to thiazide diuretics, those with (Met235 thr) angiotensinogen to ACE inhibitor and calcium channel blocker (CCB), and those with specific G-protein genes impart response to beta-blockers (BBs) and diuretics10
A number of syndromes represent genetic mutations of hypertension single-gene forms including glucocorticoid remedial hypertension (chimeric gene formation; autosomal dominant), 11-β-hydroxylase (mutation in gene encoding), 17-α-hydroxylase deficiency, Liddle syndrome (mutation in the sodium channel gene), hypertension exacerbated by pregnancy, syndrome of apparent mineralocorticoid excess, and pseudohypoaldosteronism.11 Also, human atrial natriuretic peptide (hANP) is an attractive gene for linking specific population groups to an associated increased risk for hypertension. More recently, polymorphisms of the angiotensinogen gene have been detected in hypertensive patients and in children of hypertensive parents.
The continued advances in molecular biology and newer technologies make likely the possibility of gene expression profiling being applied to hypertensive research, diagnosis, and treatment selection in the future.12
SIGNIFICANCE OF SYSTOLIC, DIASTOLIC, AND PULSE PRESSURE
A shift in diagnostic emphasis from diastolic BP to systolic BP has occurred beginning in the 1990s.13–15 A reanalysis of the Framingham Heart Study with longer follow-up data and more extensive cardiovascular data tracking showed that at all levels of systolic pressure (even within a normal range), the height of the systolic BP accurately predicted coronary heart disease (CHD).16 In addition, these data also suggest that the pulse pressure (systolic BP–diastolic BP) is a major independent predictor of CHD. A wide pulse pressure is a marker for large artery stiffness and for vascular aging (arteriosclerosis). Elevated coronary arterial calcification scores are associated with arterial stiffness and increased pulse pressure.17 Age is a determinant of the importance of pulse pressure in hypertension. A growing body of evidence supports pulse pressure readings as an important predictor in patients >65 years of age.18,19 Furthermore, pulse pressure may be a strong predictor of CV risk in the presence of compromised ventricular function with normal or low systolic BP.20
Therefore, systolic BP, diastolic BP, and pulse pressure are important in staging hypertension at different ages. Earlier generations of physicians favored the importance of diastolic BP over systolic BP, in part because hypertension was apparently a young person’s disease. With the aging of the population, hypertension has become a disease of older patients specifically reflected by isolated systolic hypertension (ISH). As arteries stiffen and pulse wave amplification decreases with aging, a general shift in elevation occurs from diastolic BP to systolic BP, and eventually in some, to pulse pressure as predictors of CV risk.21
There are patients in whom pulse pressure does not represent arterial stiffness (discrepancy between central and brachial pulse pressure, mild arterial stiffness, increased cardiac output, variable heart rate, and vasodilation). Moreover, pulse pressure cannot replace systolic BP as a single measure of CHD risk. Systolic BP and diastolic BP together are frequently superior to systolic BP alone in predicting CV risk. From a practical standpoint, physicians should first measure systolic BP (especially in healthy middle-aged and elderly cohorts) and then adjust risk upward for pulse pressure if there is a discordantly low diastolic BP (postmyocardial infarction, heart failure, end-stage renal disease [ESRD], etc.). Only when there is a discordantly low diastolic BP does pulse pressure add to systolic BP in predicting CV risk.
EVALUATION OF HYPERTENSION
A complete history, physical examination, basic serum chemistries, urinalysis, and electrocardiogram (ECG) are recommended for the initial evaluation of a hypertensive patient. Urinalysis is especially important because of the impact that renal disease has on both treatment selection and target goals for BP lowering.
The patient’s history should include a detailed family history, notation of early cerebrovascular hemorrhagic stroke (if <60 years old), nonprescription medications (nonsteroidal anti-inflammatory drugs, diet pills, decongestants, appetite suppressants, herbal therapy), birth control pills, alcohol/street drugs, and sleep history. The physician should always be alert to history or physical exam findings that suggest a secondary cause for the hypertension.
The physical examination should include two or more BP measurements separated by 2 minutes, with the patient either supine or seated, and after standing for at least 2 minutes, in accordance with recommended techniques. BP should be verified in the contralateral arms; if values are different, the higher value should be used. Measurements of height, weight, and waist circumference should be obtained. Special attention should be directed to the funduscopic examination; the presence or absence of carotid bruits or distended neck veins, thyroid enlargements; and examination of the heart, lungs, abdomen, and extremities. Particular attention should be directed to peripheral pulses, presence of abdominal bruits, and presence or absence of edema. A neurologic assessment should also be performed.
The presence of significant arteriosclerosis or arterio-venous (AV) nicking on funduscopic examination indicates in most cases that the BP has been elevated for >6 months. Arteriolar changes are the most common manifestation of hypertensive retinopathy. The mean ratio of arteriole-to-venular diameter in nonhypertensive patients is 0.84. This ratio progressively decreases with increased mean arterial BP. AV nicking can be detected where branch retinal arteries cross over veins. The thickened arteriole wall compresses the thin-walled vein, causing a tapering or “nicked” appearance.
A basic laboratory evaluation should include a urinalysis, microalbuminuria measurement, complete blood count, blood chemistry (potassium, sodium, creatinine, fasting glucose, uric acid), a full fasting lipid profile, and an ECG. An elevated uric acid value may predict the development of hypertension, is frequently present in patients with hypertension, and the degree of elevation also correlates with the degree of BP elevation. Uric acid may also have a pathogenic role in progressive renal disease.
Ambulatory blood pressure monitoring (ABPM), an echocardiogram, and assessment of plasma renin activity (PRA) are not indicated for routine evaluation of most hypertensive patients at the first visit. Recent European guidelines for hypertension have emphasized the importance of estimating the degree of underlying arterial disease by measuring arterial brachi index (ABI) and pulse wave velocity (PWV) when available, to better stratify patients at risk for cardiovascular complications and to tailor aggressive antihypertensive therapy for these individuals.
ABPM is currently considered the gold standard for measuring BP accurately. An average 24 hour BP of ≥130/80 mm Hg is considered diagnostic of hypertension. Based on 24 hour ABPM results and office BP readings, several patterns of BP have been identified which that may have a bearing on managing patients with hypertension (see Fig. 52.1.). Individuals with white coat hypertension have persistently elevated office BP (≥140/90 mm Hg) and normal 24-hours ABPM (<130/80 mm Hg). Data are not yet clear on the clinical significance of white coat hypertension and its impact on TOD and cardiovascular outcomes. In current clinical practice, identifying patients with white coat hypertension helps in reducing overtreatment of these individuals with antihypertensive medications and avoiding iatrogenic complications including hypotension. Masked hypertension is defined as persistently normal or controlled office BP measurements with elevated outside office BPs. The prevalence of masked hypertension ranges from 8% to 16% based on the population sampled. Preliminary studies indicate that the burden of TOD in these individuals may be similar to that of patients with essential hypertension. Nocturnal hypertension (average night-time BP of >120/70 mm Hg) is a subgroup of masked hypertension and was recently demonstrated to be high in prevalence among African Americans with hypertension and chronic kidney disease (CKD). There are no expert guidelines available yet regarding the management of masked hypertension.
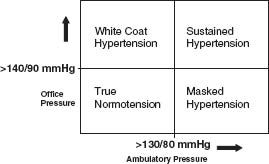
FIGURE 52.1 Patterns of blood pressure which may have a bearing on managing patients with hypertension.
Higher ambulatory systolic or diastolic BP predicts CV events even after adjustment for classic risk factors.22 Data from clinical trials that treated patients based on office BP readings demonstrate that individuals who had better average 24-hour BP control during the study had improved cardiovascular outcomes.
A major drawback in the routine use of 24-hour ABPM is that it is expensive and not reimbursed by health insurance plans. Home blood pressure monitoring (HBPM), if performed correctly, is a much cheaper alternative and provides similar accuracy to 24-hour ABPM in managing patients with hypertension. Guidelines on HBPM were recently released by the American Heart Association and American Society of hypertension and have recommended a cutoff of 135/85 mm Hg to define hypertension. A major drawback of HBPM when compared to 24-hour ABPM is the lack of evaluation of BP during sleep.
Resistant hypertension is defined as the persistence of BP >140/90 mm Hg while being treated with a rational triple-drug therapy, optimally including a diuretic. In addition, patients with hypertension who require four or more antihypertensive medications to control BP are also termed to have refractory hypertension. Resistant hypertension falls into two broad categories: apparent resistance and true resistance (Table 52.1).23
TABLE
52.1 Causes of Refractory Hypertension
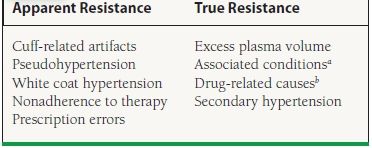
aObesity, insulin resistance, ethanol excess, sleep apnea.
bDrug–drug interactions and specific drugs that may produce refractory hypertension include: nonsteroidal antiinflammatory drugs (NSAIDs), sympathomimetic drugs (decongestants, appetite suppressants), corticosteroids, chlorpromazine, over-the-counter dietary substances (i.e., ephedra, rehung, bitter orange), tricyclic antidepressants, cocaine, amphetamines, cyclosporine, tacrolimus, erythropoietin, anabolic steroids, monamine oxidase inhibitors, oral contraceptives, licorice, and some chewing tobaccos.
The exact prevalence of resistant hypertension is unknown. Clinical trials suggest that it is not rare, involving perhaps 20% to 30% of study participants. Review of clinical records of patients seen in an outpatient setting demonstrates resistant hypertension to be present in approximately 10% of patients in a primary care setting and in more than 30% of patients in subspecialty clinics. Patient noncompliance and suboptimal therapeutic regimens are the major causes for apparent resistant hypertension (Fig. 52.2).24 More intensive therapy with emphasis on targeted volume control using diuretic therapy can achieve goal BP levels in a significant percentage of patients with apparent resistant hypertension.25
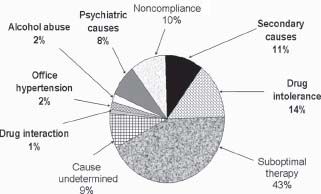
FIGURE 52.2 Of 436 patients treated at a hypertension clinic, 92 (21%) had refractory hypertension. In 83 patients, a cause was identified, the most frequent being suboptimal therapy. BP was brought under control or improved in 58 patients. (Data from Yakovlevitch M, Black HR. Resistant hypertension in a tertiary care clinic. Arch Intern Med. 1991;151:1786–1792.)
Awareness of the association among sleep-disordered breathing, sleep apnea, and hypertension has increased over the past few years.26 Both hypoxia and CO2 retention excite central and peripheral chemoreceptors activating the renin–angiotensin system, which can lead to vasoconstriction and increased BP. Typically, the onset of sleep is associated with a significant decrease in BP of 10% to 20% in normotensive individuals. Patients with disrupted sleep patterns do not experience a nocturnal dip in BP. When treated with cPAP, the nocturnal dip in BP is restored (Fig. 52.3).27
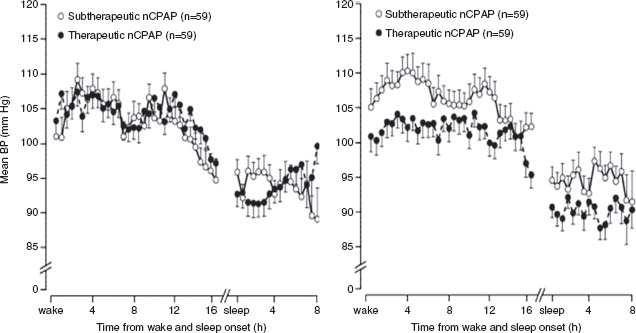
FIGURE 52.3 Randomized trial comparing treated (therapeutic vs. subtherapeutic CPAP (1 cm H2O over a 1-month period) and untreated men with sleep apnea. Bars are standard errors for every 30-minute period, synchronized to wake and sleep times. (Reprinted from the Lancet, 359, Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial, 204–210, © 2002, with permission from Elsevier.)
Novel therapies are being developed to improve BP control in patients with resistant hypertension. Some recent reports indicate a high prevalence of primary aldosteronism in patients with resistant hypertension and others have demonstrated that mineralocorticoid receptor (MR) antagonists provide significant antihypertensive benefit when added to existing multidrug regimens. Catheter-based renal denervation for treatment-resistant hypertension appears to be another novel approach. Results of a recent multicentre, prospective, randomized trial demonstrate that a significantly higher percentage of patients with drug-resistant hypertension who underwent renal denervation had improved BP control when compared to those receiving usual medical therapy.
CLINICAL APPROACHES TO HYPERTENSION
Contrary to results from National Health and Nutrition Examination Survey (NHANES) surveys in the past, which demonstrated an increasing prevalence of hypertension in the United States, results of the latest NHANES show that the prevalence of hypertension has remained at 29% over the past decade. Results from this survey also demonstrated that BP control (achieving a target BP of <140/90 mm Hg) has improved from 27.3% in 1988 to 1994 period to 50.1% in 2007 to 2008. Hypertension prevalence is highest among non-Hispanic blacks and women, and increases with age and elevated body mass index (BMI). Interestingly, hypertension control is lower in younger individuals (18 to 39 years) as compared to older individuals.28
Several landmark clinical trials have assessed the impact of different therapeutic agents on outcome in the presence of hypertension. These studies highlight the importance of treatment selection in the individual hypertension patient.29 These trial findings, coupled with the recommendations of the Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC VII),30,31 underscore the importance of recognizing up-to-date BP classification, selecting the appropriate agents for the clinical setting and achieving effective target BP lowering.
The current classification of BP for adults 18 years of age or older in JNC VII defines a prehypertension category that precedes stages I and II.32 When considering the number of patients with a BP of 120 to 139/80 to 89 mm Hg, prehypertension represents a major public health problem. Vigorous attempts at lifestyle modifications should be undertaken for individuals categorized as prehypertensive. Patients with systolic BPs between 120 and 140 mm Hg are not entirely free from a potential CV event; these prehypertensive individuals have a higher risk for developing hypertension than those with systolic BPs < 120 mm Hg. Figure 52.4 shows the importance of matching the initial drug selection with the stage of hypertension and the presence or absence of compelling indications (heart failure, diabetes mellitus type 1 or 2, proteinuria, renal disease, isolated hypertension, myocardial infarction, etc.). In the JNC VII, there was emphasis on attention to antihypertensive drug selection as well as intense BP control (defined as target BP <130/80 mm Hg) in the presence of compelling indications including diabetes mellitus, CKD with proteinuria, and congestive heart failure (CHF). However, recent results from two large prospective clinical trials, Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial and African-American Study of Kidney Disease and Hypertension (AASK), have brought into question the premise that intense BP lowering in these high-risk goups is beneficial. In the ACCORD trial, lowering of systolic BP to a goal of 120 mm Hg (intense BP-lowering group) in patients with diabetes was not associated with a reduction in cardiovascular outcomes when compared with the standard therapy group (systolic blood pressure [SBP] goal of 140 mm Hg). However, the risk of stroke was reduced in the intense BP-lowering group. The overall analysis of the follow up study of AASK participants (African American patients with CKD and hypertension) who were randonmized to intense and usual/standard BP-lowering goals did not show any benefit with intense BP lowering in these patients. However, for individuals with an elevated urine protein-to-creatinine ratio (>0.22), more intensive therapy, with a BP goal/target of approximately 130/80 mm Hg, appeared to reduce the likelihood of death or progression to ESRD.
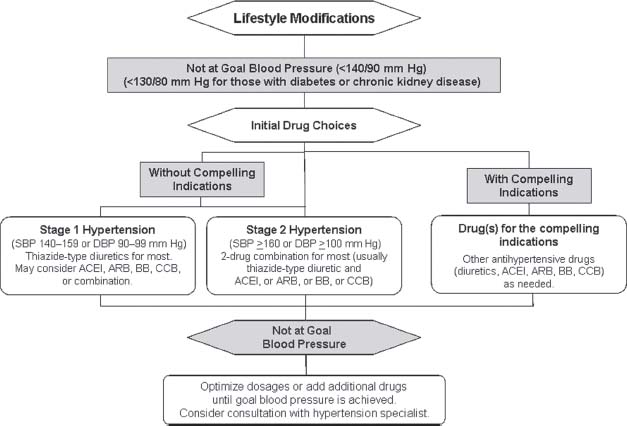
FIGURE 52.4 Algorithm for treatment of hypertension, based on randomized controlled trials. SBP, systolic blood pressure; DPB, diastolic blood pressure; ACE, angiotensin-converting enzyme; ARB, angiotensin-II receptor blocker; CCB, calcium channel blocker; HYTN, hypertension. (Adapted from Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359:204–210; Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. JAMA. 2003;289:2560–2571.)
Findings from recent clinical trials (Table 52.2) point out specific caveats for therapeutic selection in high-risk patients for CVD,33 for those with diabetic renal disease,34,35 and in high-risk ethnic groups (e.g., African Americans).36 For patients with essential hypertension who are at high risk for CVD, the use of diuretic therapy resulted in outcomes at least equivalent to the use of ACE inhibitors or CCBs in the ALLHAT study.37 Dihydropyridine calcium blockers should not be used as monotherapy in patients with proteinuric renal disease, whether associated with diabetes mellitus or hypertension. The role of BBs, especially those without vasodilating properties such as atenolol, in the management of hypertension in the absence of compelling cardiac indications is still under debate and will need further clarification. Recent clinical trials with metoprolol and newer vasodilating BBs (carvedilol and bucindolol) have shown benefit in CHF patients when added to standard therapy including ACE inhibitors.38,39 For patients with type 1 diabetes, ACE inhibitor therapy is the cornerstone of treatment. ACE inhibitors and angiotensin-II receptor blockers (ARBs) have demonstrated favorable results in both diabetic and nondiabetic renal disease. The greatest benefit for slowing progression of type 2 diabetes with renal disease can be seen with ARBs, based on findings from the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) and Irbesartan Diabetic Nephropathy Trial (IDNT) studies.40,41 Aliskiren belongs to a new class of antihypertensive medication that act by direct renin inhibition (DRI). In clinical trials, BP reduction by aliskiren was equivalent to ARBs including valsartan, irbesartan, and losartan. Several trials have examined the effect of aliskiren in combination with other antihypertensive drugs. Aliskiren when used in combination with atenolol showed a significantly greater reduction in diastolic BP (14.1 mm Hg) when compared with aliskiren monotherapy (11.3 mm Hg) but not with atenolol alone (13.7 mm Hg).12 The additive effects of a high-dose combination of aliskiren and valsartan demonstrated that the combination reduced BP by a mean of 17.2/12.2 mm Hg, a greater reduction than was observed with either component (aliskiren, 13.0/9.0 mm Hg; valsartan, 12.8/9.7 mm Hg; p < 0.0001). The Aliskiren in the Evaluation of Proteinuria in Diabetes (AVOID) study evaluated the additive effect of aliskiren in patients with hypertension and type 2 diabetes with nephropathy who were on an ARB (losartan). The study arm that received a combination of aliskiren and losartan had a significant reduction in albuminuria, a surrogate end point for renoprotection. Direct renin inhibitors offer an additional choice in the current armamentarium of antihypertensive medications, to treat those with hypertension and provide renoprotection in those with diabetic nephropathy. Data on the effects of DRI on cardiovascular and renal end points are lacking and need to be studied further before they can be considered a first-line agent in treating patients with compelling indications.
TABLE
52.2 Summary of Cardiovascular and Kidney Outcome Trials (2001–2010)
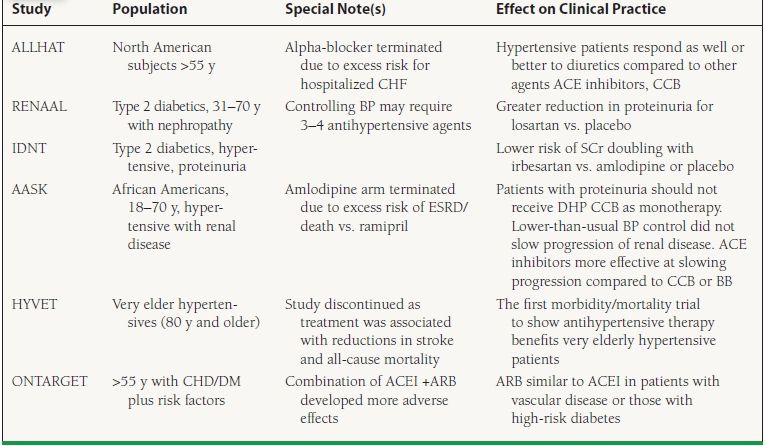
y, year(s); CHF, congestive heart failure; ACE, angiotensin-converting enzyme; CCB, calcium channel blocker; SCr, serum creatinine; ESRD, end-stage renal disease; DHP CCB, dihydropyridine calcium channel blocker; BP, blood pressure. Trial names: ALLHAT, Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial; RENAAL, Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan; IDNT, Irbesartan Diabetic Nephropathy Trial; AASK, African American Study of Kidney Disease and Hypertension; HYVET, Hypertension in the Very Elderly Trial; ONTARGET, Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial.
Because patients with CKD (serum creatinine ≥1.4 mg/dL or estimated glomerular filtration rate [eGFR] <60 mL/min) are more likely to die from CVD than from ESRD, hypertension should be aggressively controlled.42 Despite this, awareness of kidney disease is low, especially with respect to other chronic diseases. Decreased CKD awareness is most prevalent in first-degree Spanish speakers, male gender, non-Hispanic blacks, and in patients with hypertension.43 The risk for CV and renal disease events starts at systolic BP levels as low as 115 mm Hg.44 ACE inhibitors or ARBs should be used in CKD patients whenever possible. An increase in baseline serum creatinine up to 35% on these agents is acceptable unless clinically resistant hyperkalemia develops. Hypertensive patients with eGFR <30 mL/min/1.73 m2 will require increasing doses of loop diuretic in combination with other agents to optimize volume, a critical determinant of elevated BP.
The development of microalbuminuria is associated with abnormal vascular reactivity, salt sensitivity, increased presence of TOD, and loss of nocturnal dipping in BP.45 An elevated urine albumin-to-creatinine ratio heralds the need for aggressive BP control.
Effective BP control can be achieved in the majority of patients with hypertension, but more than two (2.7 to 3.8) medications may be needed to reach target BP levels.46 When BP is >20/10 mm Hg above target, consideration should be given to initiating therapy with two drugs. Extensive clinical experience with available antihypertensive agents suggests that any single drug preparation will control only 30% to 65% of patients treated, whereas the addition of a second or third drug to the regimen can improve control rates into the range of 90% to 95%. Patients should return for follow-up monthly until the BP goal is achieved. Serum potassium and creatinine should be monitored at least twice per year.47
Among older persons, systolic BP is a better predictor of events (CHD, CVD, heart failure, stroke, ESRD, and all-cause mortality) than diastolic BP. The initial treatment goal in older patients should be the same as in younger individuals, namely, to achieve a BP below 140/90 mm Hg. However, the concept of a J curve for mortality with exaggerated BP lowering may be of greatest importance in the elderly. Findings from both the Systolic Hypertension in the Elderly Program (SHEP) trial48 and the Rotterdam Study49 suggest that there may be an increased CV risk in the lowest strata of BP and that reducing systolic BP to <130 mm Hg or diastolic BP to <65 mm Hg may not represent optimal strategy in the elderly.
Electrocardiographic or echocardiographic evidence of left ventricular hypertrophy (LVH) is associated with increased risk of coronary disease, ventricular dysrhythmias, and sudden death50,51 and requires optimal target BP. Most of the antihypertensive drugs used for initial hypertension therapy induce regression of LVH. In the losartan intervention for endpoint reduction trial, ARBs were superior to BB therapy in reducing CV endpoints in hypertensive patients with electrocardiogram evidence of LVH.
Because the risk of heart disease and stroke increases with age among women, increasing attention has been focused on the nearly 30 million American women over age 50 years. In women 50 to 64 years of age, 47% have high BP—this figure increases to 58% in women 65 to 74 years of age, and to 75% for those 75 years and older. African American women have a death rate from hypertension that is approximately 4.5 times higher than the rate for white women, which makes hypertension the probable cause of up to 20% of all deaths in hypertensive African American women. There is no evidence that women respond any differently than men to the risk-reduction effect of antihypertensive drugs,52 and the JNC VII guidelines should be applied equally to women.
SECONDARY HYPERTENSION
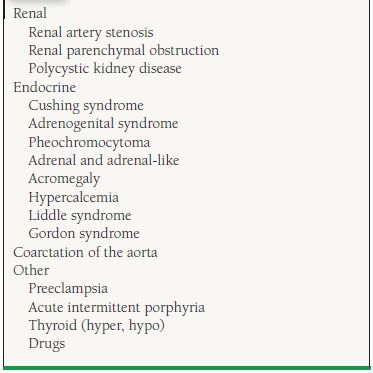
For hypertensive patients who are resistant to treatment with two or more agents, a number of clinical clues can suggest the possible presence of secondary hypertension. Table 52.3 divides the causes of secondary hypertension into four broad categories. Secondary hypertensive disorders can be effectively treated or cured, leading to partial or complete normalization of resistant hypertension in most patients.
TABLE
52.3 Clues to Secondary Hypertension
Primary Aldosteronism
Primary aldosteronism is the most common cause of hypertension due to an endocrinopathy. The most common cause of primary aldosteronism is an aldosterone-producing adenoma (70% to 80%). However, glucocorticoid-remediable aldosteronism (GRA), adrenal hyperplasia, and adrenal carcinoma are other considerations. Although the clinical manifestations of primary aldosteronism are not distinctive, the best clues to the presence of primary aldosteronism include hypertension with spontaneous hypokalemia (<3.5 mEq/L), hypertension with provoked hypokalemia (<3.0 mEq/L during diuretic therapy), and hypertension with difficulty in maintaining normokalemia despite potassium supplementation.
Primary aldosteronism should be considered in any patient with both refractory hypertension and hypokalemia with inappropriate kaliuresis (urine potassium >30 mEq/L per 24 hours). One should be especially suspicious of primary aldosteronism if potassium is <3.5 mEq/L despite potassium supplementation, ACE inhibitor or ARB therapy, and/or a potassium-sparing diuretic. In addition, patients may develop muscle spasms, periodic paralysis, or metabolic alkalosis. The clinician needs to remember that not all patients with primary aldosteronism have hypokalemia; 7% to 38% of patients with primary aldosteronism may have normal serum potassium.53 Even 10% to 12% patients with positive tumors may not have hypokalemia during short-term salt loading. Individuals with hypertension and renal potassium wasting can be differentiated into high-renin and low-renin states (Table 52.4). Usually, the plasma renin concentration is <1 ng/mL/h in mineralocorticoid excess, and fails to rise above 2 ng/mL/h after salt depletion and upright posture.
TABLE
52.4 Biochemical Classification of Patients with Hypertension, Hypokalemia, and Renal Potassium Wasting
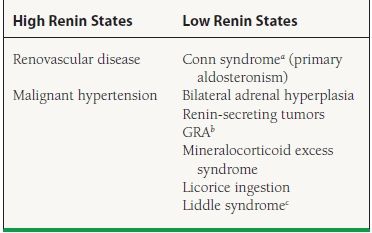
aAldosterone-secreting adrenal adenoma.
bChildren, early-onset severe hypertension, history of early hemorrhagic stroke, adrenocorticotropic hormone (ACTH) and renin–angiotensin system is suppressed. Suppression of ACTH with glucocorticoids decreases aldosterone and cures the hypertension. Diagnose by high 18-hydroxycortisol/18-oxycortisol.
cHypertension, decreased potassium, alkalosis, decreased aldosterone, and sodium channel mutation.



