(1)
University of Ottawa The Ottawa Hospital, Ottawa, ON, Canada
Results that dictate a reduction in end points observed in a few hallmark and other recent randomized clinical trials (RCTs) are given. The appropriate reference source is given so that the reader can explore the details of the RCT if required. Because of the constraints of available space, only a brief comment is given in the text regarding the results and their implications.
Prior to the assessment of these RCTs, the reader may wish to review the following brief points that relate to the interpretation of clinical trials:
Questions to ask when reading and interpreting the results of a clinical trial include (Guyatt et al. 1994)
The “gold standard” statistical test of treatment effect is the determination of the p value using the χ 2 or Fisher’s exact test. Although a p value <0.05 is at the significant level, in clinical medicine it is advisable to use p ≤ 0.02 as a level of significant treatment effect.
Statements describing treatment effect (Antman et al. 2002): relative risk (RR), relative risk reduction, odds ratio (OR), and absolute risk difference (ARD).
An RR, relative risk reduction, or OR < 1 indicates treatment benefit. However, an RR reduction may give the impression of a greater treatment effect if the ARD and number needed to treat, the (1/ARD), are not given: the number of lives saved per 1,000 patients treated.
Meta-analysis: Data pooling or quantitative review provides partial answers that may receive support from some and skepticism from others. One rule must be invoked when one is assessing the results derived from clinical meta-analysis: do not accept information based on subgroup analysis unless the primary end point, particularly the effect on all-cause mortality or cardiovascular mortality, showed a significant treatment effect.
Are the results of the study valid? Was the assignment of patients to treatment randomized? Were all patients who entered the trial properly accounted for at its conclusion? Was follow-up complete and patients analyzed in the groups to which they were randomized?
Acute Coronary Syndrome RCTs
ATLAS ACS 2–TIMI 51: Rivaroxaban for ACS
In this double-blind, placebo-controlled trial, the ROCKET investigators randomly assigned 15,526 patients with a recent acute coronary syndrome (~STEMI ~ 50 %, NSTEMI 25 %, unstable angina %) to receive twice-daily doses of either 2.5 mg or 5 mg of rivaroxaban or placebo for a mean of 13 months and up to 31 months. The primary efficacy end point was a composite of death from cardiovascular causes, myocardial infarction, or stroke (Mega et al. 2012).
Enrollment occurred within 7 days after hospital admission for an ACS. Subjects received the first dose of study drug no sooner than 4 h after the final dose of IV heparin, 2 h after the final dose of bivalirudin, and 12 h after the final dose of other intravenous or subcutaneous anticoagulants (e.g., enoxaparin or fondaparinux).
Rivaroxaban significantly reduced the primary efficacy end point of death from cardiovascular causes, myocardial infarction, or stroke, as compared with placebo, with rates of 8.9 % and 10.7 %, respectively, p = 0.008. The twice-daily 2.5-mg dose of rivaroxaban reduced the rates of death from cardiovascular causes (2.7 % versus 4.1 %, p = 0.002) and from any cause (2.9 % versus 4.5 %, p = 0.002), a survival benefit that was not seen with the twice-daily 5-mg dose.
In addition, rivaroxaban reduced the risk of stent thrombosis (Mega et al. 2012).
The reduction in the primary efficacy end point with rivaroxaban was consistent among the subgroups except for patients with a history of stroke or transient ischemic attack (TIA). Thus the agent including apixaban and other Xa inhibitors should be avoided in patients with prior stroke or TIA.
The 5-mg dose of rivaroxaban, as compared with placebo, did not significantly reduce the risk of death from either cardiovascular causes or any cause and differed significantly from the 2.5-mg dose of rivaroxaban (p = 0.009 for both comparisons) (Mega et al. 2012).
There was no significant difference in the rates of fatal bleeding associated with rivaroxaban as compared with placebo. The lower dose resulted in significantly lower rates of TIMI minor bleeding [0.9 % versus 1.6 %, and fatal bleeding (0.1 % versus 0.4 %, p = 0.04)]. Liver abnormalities were similar among patients treated with rivaroxaban or placebo. The advantages of the addition of rivaroxaban were observed regardless of whether patients presented with a STEMI, NSTEMI, or unstable angina (Mega et al. 2012).
This advantage was observed regardless of PCI or non-PCI therapy.
A similar RCT with the Xa inhibitor apixaban (ARISTOTLE:Granger et al 2011), which was efficacious for atrial fibrillation (See section on AF trials), was shown not effective and caused excessive bleeding but doses of the drug were obviously too high.
Bivalirudin for ACS
Acuity: The Acute Catheterization and Urgent Intervention Triage Strategy RCT evaluated the use of bivalirudin as a replacement for UF heparin and LMWH administered in the emergency department and continuing through the catheterization laboratory. The control arm of ACUITY comprised patients treated with the heparins combined with platelet glycoprotein (GP) IIb/IIIa receptor blockers. A second arm studied bivalirudin with added GP IIb/IIIa receptor blockers; the third arm studied bivalirudin alone as monotherapy. (In the third arm, approx. 7 % of study patients received GP receptor blockers.)
Patients who received the combination of bivalirudin with GP IIb/IIIa blockers (compared with a heparin-based regimen) had equivalent rates of bleeding and ischemia; overall patient outcomes were also equivalent.
Bivalirudin monotherapy suppressed ischemic complications just as effectively as heparins plus GP IIb/IIIa blockers but was associated with half of the major bleeding, resulting in a significant improvement in overall patient outcomes. The trial was open labeled and had many flaws but provided important information for further trials with this hallmark thrombin inhibitor.
HORIZONS-AMI (2011) was a prospective, open-label, randomized trial in patients with acute STEMI, within 12 h after the onset of symptoms, and who were undergoing PCI. Patients were randomly allocated 1:1 to receive bivalirudin or heparin plus a glycoprotein IIb/IIIa inhibitor (GPI).
Results at 3 years: Compared with 1,802 patients allocated to receive heparin plus a GPI, 1,800 patients allocated to bivalirudin monotherapy had lower rates of all-cause mortality (5.9 % versus 7.7 %, difference −1.9 % p = 0.03), cardiac mortality (2.9 % versus 5.1 %, p = 0.001), and reinfarction (6.2 % versus 8.2 %, p = 0.04).
Major bleeding not related to bypass graft surgery was significantly reduced (6.9 % versus 10.5 %, p = 0.0001). There were no significant differences in ischemia-driven target vessel revascularization, stent thrombosis, or composite adverse events (Stone et al. 2011). Compared with 749 patients who received a bare-metal stent, 2,257 patients who received a paclitaxel-eluting stent had lower rates of ischemia-driven target lesion revascularization (9.4 % versus 15.1 %, p < 0.0001) after 3 years, with no significant differences in the rates of death, reinfarction, stroke, or stent thrombosis. Stent thrombosis was high (≥4.5 %) in both groups (Stone et al. 2011).
ISAR-REACT 4 Trial: Abciximab and Heparin versus Bivalirudin for non-ST-Elevation Myocardial Infarction: Subjects were all treated with ASA and clopidogrel 600 mg. Bivalirudin was administered to 860 and abciximab to 861 patients. A strategy of early invasive intervention (within 24 h after admission) was the standard of care at all centers for patients presenting with an ACS and elevated biomarker levels. All the patients in the trial were given 325–500 mg of aspirin and 600 mg of clopidogrel before they received a study drug. Before the guide wire had crossed the lesion, patients who were assigned to the abciximab group received a bolus dose of 0.25 mg of abciximab per kilogram of body weight, followed by an infusion of 0.125 μg of abciximab per kilogram per minute (maximum of 10 μg/min) for 12 h, and a bolus dose of 70 U of heparin per kilogram. Patients in the bivalirudin group received a bolus dose of 0.75 mg of bivalirudin per kilogram, followed by an infusion of 1.75 mg/kg/h for the duration of the procedure (Kastrati et al. 2011).
The primary end point occurred in 94 patients (10.9 %) in the abciximab group and 95 patients (11.0 %) in the bivalirudin group (p = 0.94).
Cumulative incidence of primary and secondary end points in the two study groups. The secondary efficacy end point occurred in 110 patients (12.8 %) in the abciximab group and 115 patients (13.4 %) in the bivalirudin group (p = 0.76). Major bleeding occurred in 40 patients (4.6 %) in the abciximab group and 22 patients (2.6 %) in the bivalirudin group (relative risk, 1.84; 95 % CI, 1.10–3.07; p = 0.02). Profound thrombocytopenia developed in 10 patients (1.2 %) in the abciximab group and in none of the patients in the bivalirudin group (p = 0.002) conclusion:
There were no significant differences in deaths, or recurrent MI, but major bleeding was ~84 % increased by abciximab (4.6 %) compared with 2.6 % for bivalirudin (Kastrati et al. 2011).
In addition advantages include a much shorter duration of IV infusion, and cost compared to the use of antiplatelet inhibitors.
Bivalirudin Alone Bests Heparin plus a GPI. In this rigorously performed double-blind RCT efficacy was similar and bleeding rates were remarkably lower with bivalirudin in patients undergoing PCI for NSTEMI (Kastrati et al. 2011).
Bivalirudin is the preferred anticoagulant agent for all PCIs.
Enoxaparin Versus Heparin
ExTRACT-TIMI 25: Enoxaparin Versus Heparin in Acute MI
Enoxaparin and Thrombolysis Reperfusion for Acute Myocardial Infarction Treatment-Thrombolysis in Myocardial Infarction (EXTRACT-TIMI) 25 RCT (Antman et al. 2006) compared enoxaparin with heparin. A total of 20,506 patients with STEMI treated with thrombolytics received enoxaparin or weight-based UF heparin for at least 48 h. The primary efficacy end point was death or nonfatal recurrent MI.
At 30-day follow-up: The primary end point occurred in 12.0 % of patients in the UF heparin group versus 9.9 % in the enoxaparin group (17 % reduction in RR; p < 0.001). Nonfatal MI occurred in 4.5 % and 3.0 %, respectively (33 % reduction in RR; p < 0.001); there was no difference in total mortality.
Major bleeding occurred in 1.4 % with UF heparin versus 2.1 % with enoxaparin (p < 0.001).
The exclusion of men with a creatinine level >2.5 mg/dL (220 μmol/L) and women with a creatinine level >2.0 mg/dL (177 μmol/L) was an important adjustment to prevent bleeding.
LMWH and Major Bleeding Advice
In patients aged > 70 years, use 0.75 mg/kg, and in all patients with creatinine clearance 30–50 mL/min dose once daily and avoid if the estimated GFR is <30 mL/min.
For the conversion of serum creatinine in mg/dL, multiply × 88 (=mmol/L). The serum creatinine is only a rough measure of renal function and must not be relied on, particularly in patients older than age 70.
Use the creatinine clearance estimated GFR rather than serum creatinine levels, but note that some electronic formulas are inaccurate in patients older than age 70 and must be adjusted in blacks (for African Americans, the laboratory-reported estimated GFR should be multiplied by a factor of 1.21 and reinterpreted accordingly).
Patients, particularly cardiac patients, older than age 70 with a serum creatinine in the upper normal range of 1–1.2 mg/dL (88–106 μmol/L) often have a lowered GFR because of the underlying normal diminution of GFR with age. A substantial number of nephrons are lost annually beyond age 70, and in many elderly patients renal disease coexists.
For patients older than age 75, enoxaparin 0.75 mg/kg once daily is an easy dose to recall, but it might be preferable to use the lower age cutoff: >70 years of age.
Enoxaparin and other LMWHs should be given once daily in patients with a GFR, creatinine clearance of 40–55 mL/min. Patients in this category received enoxaparin twice daily in several RCTs; unfortunately, patients with a creatinine clearance <30 mL/min were given 1 mg/kg once daily in some RCTs.
Importantly, if major bleeding is to be minimized in patients with ACS, LMWH should be avoided if the creatinine clearance is <30 mL/min. An estimated GFR of <30 mL/min reflects poor renal function. Clinicians most often use a creatinine clearance <15 mL/min to indicate severe renal failure. This information is correct, but end-stage renal failure (GFR < 15 mL/min) should be regarded as very severe renal failure, and drugs that have a potential to cause major bleeding must be used only with justification. Use in patients with ACS is not justifiable because alternative therapies are available.
Do not switch from UF heparin to LMWH and vice versa in the management of a patient with ACS.
Enoxaparin or Unfractionated Heparin for PCI
In a randomized open-label trial, patients presenting with STEMI were randomly assigned (1:1) to receive an intravenous bolus of 0.5 mg/kg of enoxaparin or unfractionated heparin before primary PCI. 910 patients were assigned to treatment with enoxaparin (n = 450) or unfractionated heparin (n = 460) (Montalescot et al. 2011).
The primary end point was 30-day incidence of death, complication of myocardial infarction, procedure failure, or major bleeding. The main secondary end point was the composite of death, recurrent acute coronary syndrome, or urgent revascularization.
Results: The primary end point (30-day incidence of death, complication of MI, procedure failure, or major bleeding) occurred in 126 (28 %) patients with enoxaparin versus 155 (34 %) patients on unfractionated heparin (p = 0.06). The incidence of death [enoxaparin, 17 (4 %) versus heparin, 29 (6 %) patients; p = 0.08], complication of MI p = 0.21; and major bleeding [20 (5 %) versus 22 (5 %); p = 0.79] did not differ between groups. Enoxaparin resulted in a significantly reduced rate of the main secondary end point [30 (7 %) versus 52 (11 %) patients; p = 0.015]. Death, complication of MI, or major bleeding [46 (10 %) versus 69 (15 %) patients; p = 0.03], death or complication of MI [35 (8 %) versus 57 (12 %); p = 0.02], and death, recurrent MI, or urgent revascularization [23 (5 %) versus 39 (8 %); p = 0.04] were all reduced with enoxaparin.
Enoxaparin provided an improvement in net clinical benefit in patients undergoing primary PCI (Montalescot et al. 2011).
Fondaparinux or Enoxaparin
OASIS-6: Effects of Fondaparinux on Mortality and Reinfarction in Patients With Acute ST-Segment Elevation Myocardial Infarction (STEMI).
OASIS-6 (2006) was an RCT of fondaparinux versus usual care in 12,092 STEMI patients. A 7–8-day course of fondaparinux was compared with either no anticoagulation or heparin (75 % received heparin for <48 h). Streptokinase was the main fibrinolytic used (73 % of those who received lytics). The primary outcome was a composite of death or reinfarction at 30 days.
Conclusion: In patients with STEMI, particularly those not undergoing PCI, fondaparinux significantly reduced mortality and reinfarction without increasing bleeding and strokes (OASIS-6 Trial Investigators 2006).
In areas and in countries where PCI is not readily available, the drug can be used with streptokinase without any form of heparin administration. In addition, the one dose of fondaparinux with no adjustments for weight is a great advance in developing or developed countries.
The addition of rivaroxaban 12 h after the final dose of fondaparinux and continued for >5 years should result in improved survival and represents a major breakthrough in the therapy for ACS.
Califf (2006) points out that a reasonable conclusion from this trial is that fondaparinux is highly beneficial in patients in whom the noninterventional approach has been predetermined and will be more preferred in settings in which the use of angiographic-based reperfusion is not routine. In addition, the absence of the need for dose adjustment is remarkable. The two large RCTs have endorsed fondaparinux as a leading antithrombotic drug in the treatment of ACS. There is no evidence that fondaparinux is inferior to either UF heparin or LMWH for the management of ACS. The reduction in bleeding in the fondaparinux group compared with the group receiving no antithrombotic therapy should give clinicians some confidence that at the dose used, fondaparinux has a desirable margin of safety (Califf (2006)). Also, heparin-induced thrombocytopenia (HIT) can be avoided. Caution with dosage is needed, however, in patients with significant renal dysfunction (estimated GFR < 50 mL/min).
Invasive Versus Conservative Strategy in ACS Patients
TACTICS-TIMI 18 evaluated the superiority of an invasive strategy (stenting and use of the platelet inhibitor tirofiban) in patients with unstable angina and non-ST-elevation MI (NSTEMI). Coronary angiography was completed within 4–48 h, and PCI was accomplished in the invasive arm (TACTICS-TIMI-18 Investigators 2001). The cumulative incidence of the primary end point of death, nonfatal MI, or rehospitalization for an ACS during the 6-months follow-up period was lower in the invasive-strategy group than in the conservative-strategy group (15.9 % versus 19.4 %; OR, 0.78; p = 0.025). Patients with positive troponin levels (i.e., NSTEMI patients) achieved the greatest benefit from an early invasive strategy (14.3 % versus 24.2 %; p < 0.001; see Fig. 22-1).
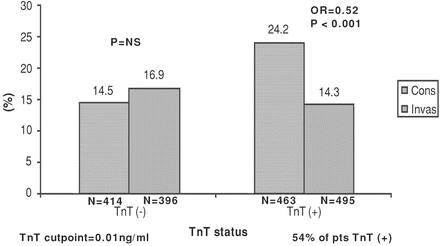

Fig. 22-1.
Outcomes of invasive and conservative strategies in TACTICS-TIMI 18 according to troponin level. Reproduced with permission from Cannon CP. Management of Acute Coronary Syndromes, 2nd ed. Humana Press, Totowa, NJ, 2003. With kind permission from Springer Science + Business Media.
Unstable angina patients were not benefited by early PCI; only patients with NSTEMI (as defined by positive troponin levels) benefited significantly.
Patients with unstable angina with or without ischemic electrocardiographic (ECG) changes and negative troponin levels showed no significant benefit with an invasive strategy.
Patients with unstable angina and negative troponin levels should not be considered to have ACS but simply unstable angina. These patients have not sustained infarctions and are a heterogeneous group with a markedly different prognosis compared with STEMI or NSTEMI patients.
TIMACS ( 2009) studied the efficacy of an early invasive strategy compared with a delayed invasive strategy. Patients were randomized to either an early invasive strategy (coronary angiography as soon as possible, followed by PCI or CABG within 24 h) or a delayed strategy (coronary angiography any time >36 h followed by PCI or CABG). A total of 3,031 patients were randomized, 1,593 to an early invasive strategy, and 1,438 to a delayed invasive strategy. About 80 % had ECG changes in keeping with ischemia (ST-segment depression of 1 mm or transient ST-segment elevation or T-wave inversion of >3 mm).
About 77 % of the patients had elevated biomarkers (NSTEMI) (Mehta et al. 2009). Thus, about 77 % were NSTEMI and only 23 % were unstable angina; the presence of these 23 % lower-risk patients dilutes the treatment effects.
The median times to coronary angiography in the early and delayed arms were 14 and 50 h, respectively. PCI was conducted more often in the early invasive group (59.6 % versus 55 %), with a median time of 16 versus 52 h; mean follow-up 6 months (Mehta et al. 2009).
Results: An early invasive strategy was not superior to a delayed invasive strategy in patients presenting with NSTEMI and unstable angina in reducing the composite end point of death and MI.
But there was a significant reduction in the incidence of the primary end point in the early invasive arm compared with the delayed invasive arm in patients with a GRACE score of >140 (the third with the highest risk); the primary outcome occurred in 13.9 % of patients in the early-intervention group, as compared with 21 % in the delayed-intervention group, a reduction of 35 % in the early-intervention group (hazard ratio, 0.65; p = 0.006) (Mehta et al. 2009).
Patients with chest pain features of MI accompanied by ST-segment depression or transient elevation and positive troponin assays (i.e., a diagnosis of NSTEMI) should undergo an early invasive intervention if facilities are readily available.
The TIMI or GRACE risk algorithms can be used effectively to predict the rates of death or MI for a year after hospitalization for ACS.
In patients with elevated troponin levels if pulmonary embolism, decompensated heart failure, severe hypertension, tachycardia, anemia, and sepsis are excluded clinically, this variable along with ECG changes (two risk factors) should establish the high-risk patient.
Fox and colleagues assessed a meta-analysis (n = 5,467 patients) designed to determine whether outcomes are improved despite trial differences. Conclusion: “a routine invasive strategy reduces long-term rates of cardiovascular death or MI and the largest absolute effect is seen in higher-risk patients. Even in intermediate risk populations, the benefits of an early invasive strategy are of similar magnitude as those aimed for and seen with current pharmacological interventions” (Fox et al. 2010).
Ticagrelor Versus Clopidogrel
PLATO: Cannon and colleagues (2010) for the platelet inhibition and patient Outcomes (PLATO) investigators compared ticagrelor with clopidogrel.
At randomization, an invasive strategy was planned for 13,408 (72 %) of 18,624 patients hospitalized for STEMI and NSTEMI. Patients were randomly assigned to ticagrelor and placebo (180 mg loading dose followed by 90 mg twice a day) or to clopidogrel and placebo (300–600 mg loading dose or continuation with maintenance dose followed by 75 mg/day) for 6–12 months. All patients were given aspirin. The primary composite end point was cardiovascular death, MI, or stroke (Cannon et al. 2010). Analyses were by intention to treat.
Results: At 1 year the primary composite end point occurred in fewer patients in the 6,732 ticagrelor-treated group than in the 6,676 clopidogrel group: 569 (event rate 9 %) versus 668 (10.7 %, hazard ratio 0.84, p = 0.0025).
Ticagrelor compared with clopidogrel significantly reduced all-cause mortality at 12 months in all patients (4.5 % versus 5.9 %, hazard ratio 0.78, p < 0.001), and in patients undergoing an early invasive strategy (3.9 % versus 5 %, p = 0.01).
There was no difference between clopidogrel and ticagrelor groups in the rates of total major bleeding 691 (11.6 %) versus 689 (11.5 %), or severe bleeding. These investigators concluded that ticagrelor seems to be a better option than clopidogrel for patients with STEMI OR NSTEMI, for whom an early invasive strategy is planned (Cannon et al. 2010).
During 12 months, dyspnea occurred more often in the ticagrelor group than in the clopidogrel group [924 (event rate 13.9 %) versus 527 (8 %); p < 0.0001]. Only 51 (0.8 %) patients in the ticagrelor group and ten (0.2 %) in the clopidogrel group permanently discontinued the study drug because of this adverse event (Cannon et al. 2010). Most episodes lasted less than a week (Wallentin et al. 2008); the levels of creatinine increased slightly more during the treatment period with ticagrelor than with clopidogrel. These minor effects can be overcome, however, by not administering the drug to patients with chronic obstructive pulmonary disease, and those with a moderate degree of renal failure or in those over age 75 because renal dysfunction may be present despite the finding of a normal serum creatinine; also, in this age group, intracranial hemorrhage is more likely to occur with potent agents that include ticagrelor and prasugrel.
Ticagrelor blocks reuptake of adenosine by red blood cells (Björkman et al. 2007), “which might explain why some patients had dyspnea and bradycardia. Inhibition of adenosine reuptake could also lead to cardiovascular benefit through reduction in blood pressure (BP), improved coronary flow, or protection against reperfusion injury”.
There was a higher incidence of ventricular pauses in the first week, but not at day 30, in the ticagrelor group than in the clopidogrel group. Pauses were rarely associated with symptoms; the two treatment groups did not differ significantly with respect to the incidence of syncope or pacemaker implantation.
Cannon and colleagues emphasized that their results show that ticagrelor might be safely initiated at first presentation and does not need to be withheld until the coronary anatomy is defined.
The PLATO investigators estimate that “use of ticagrelor instead of clopidogrel for 1 year in 1,000 patients with acute coronary syndromes and who are planned to undergo an invasive strategy at the start of drug treatment would lead to 11 fewer deaths, 13 fewer MIs, and six fewer cases of stent thrombosis without an increase in the rates of major bleeding or transfusion” (Cannon et al. 2010).
Stone, in an editorial, emphasized that:
Unlike clopidogrel and prasugrel, which bind irreversibly to the platelet surface-membrane (P2Y12) receptor, ticagrelor is a reversible P2Y12 receptor blocker, with platelet function returning to normal, 2–3 days after discontinuation (Gurbel et al. 2009) compared with 5–10 days after discontinuation of clopidogrel and prasugrel. The reversible action of ticagrelor is of paramount importance “in mitigating bleeding,” thus allowing coronary bypass graft surgery, soon after drug discontinuation. In addition within 30 min, a ticagrelor loading dose of 180 mg results in roughly the same level of inhibition of platelet aggregation as that achieved 8 h after a clopidogrel loading dose of 600 mg (Gurbel et al. 2009). Compelling results support ticagrelor as a new standard of care in acute coronary syndromes (Stone 2010).
Ticagrelor (Brilinta) FDA approved blood-thinning drug Brilinta to treat acute coronary syndromes. Boxed warning says daily aspirin doses above 100 mg decrease effectiveness. In clinical trials, Brilinta was more effective than Plavix in preventing heart attacks and death, but that advantage was seen with aspirin maintenance doses of 75–100 mg once daily,
In the PLATO trial, ticagrelor compared with clopidogrel consistently reduced the primary end point and its components CV death and MI, without a difference in overall major bleeding in patients with an entry diagnosis of NSTEMI including in-hospital medically managed (Lindholm et al. 2013).
IV Metoprolol Studies
In patients with anterior Killip class < or equal to 11 ST-elevation MI undergoing PCI, early IV metoprolol before reperfusion resulted in higher long-term left ventricular ejection fraction. This administration reduced the incidence of severe left ventricular dysfunction and implantable cardioverter defibrillator indications and fewer admissions for heart failure (Pizarro et al. 2014).
COMMIT/CCS-2: Second Chinese Cardiac Study (CCS-2) (31)
This trial was conducted on the emergency treatment of STEMI patients. Patients received aspirin and were randomized to receive clopidogrel 75 mg/day or placebo; within these two groups, patients were then randomized to metoprolol (15 mg IV in three equal doses followed by 200 mg/day oral) or placebo. Patients were randomized within 24 h of suspected acute MI and demonstrated ST elevation or other ischemic abnormality and excluded if they were undergoing PCI. The primary end point varied between study drugs: for clopidogrel, it was death or the combination of death, reinfarction, or stroke up to 4 weeks in the hospital or prior to discharge; for metoprolol, it was death or death, reinfarction, or cardiac arrest/ventricular fibrillation (VF) up to 4 weeks in the hospital or prior to discharge.
Metoprolol produced a significant 18 % relative risk reduction in reinfarction (2.0 % versus 2.5 %; p = 0.001) as well as a 17 % relative risk reduction in ventricular fibrillation (2.5 % versus 3.0 %; p = 0.001); there was no effect on mortality (7.7 % versus 7.8 %). Metoprolol significantly increased the relative risk of death from cardiogenic shock by 29 %, with the greatest risk of shock occurring primarily on day 0–1 (COMMIT/CCS-2 2005).
Cardiogenic shock understandably was more evident in patients in Killip classes II and III; this adverse effect was largely iatrogenic because the dose of metoprolol was excessive. Oral beta-blocker therapy is preferred, and the IV use is cautioned against.
Importantly. metoprolol IV was administered to patients who were hemodynamically unstable, a situation that must be avoided. (See Chap. 2 , Beta-Blocker Controversies.)
The METOCARD-CNIC
Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction trial
The study randomized 270 patients Killip class II anterior STEMI presenting early after symptom onset (<6 h) to pre-reperfusion IV metoprolol or control group. Long-term magnetic resonance imaging (MRI) was performed on 202 patients (101 per group) 6 months after STEMI. Patients had a minimal 12-month clinical follow-up (Pizarro et al. 2014).
“Results Left ventricular ejection fraction (LVEF) at the 6 months MRI was higher after IV metoprolol (9.9 % versus 11.7 % in control subjects p ¼ 0.025). The occurrence of severely depressed LVEF (35 %) at 6 months was significantly lower in patients treated with IV metoprolol (11 % versus 27 %, p ¼ 0.006). The proportion of patients fulfilling Class I indications for an implantable cardioverter-defibrillator (ICD) was significantly lower in the IV metoprolol group (7 % versus 20 %, p ¼ 0.012). At a median follow-up of 2 years, occurrence of the pre-specified composite of death, heart failure admission, reinfarction, and malignant arrhythmias was 10.8 % in the IV metoprolol group versus 18.3 % in the control group. Heart failure admission was significantly lower in the IV metoprolol group” (Pizarro et al. 2014).
In patients with anterior Killip class II STEMI undergoing pPCI, early IV metoprolol before reperfusion resulted in higher long-term LVEF, reduced incidence of severe LV systolic dysfunction and ICD indications, and fewer heart failure admissions (Pizarro et al. 2014).
The results of the COMMIT (2005), which showed no short-term net clinical benefit of early metoprolol in STEMI patients undergoing thrombolysis, discourage some from using the drug. But in that study the high dose of metoprolol given to patients with a systolic blood pressure <110 mmHg was inappropriate (Khan 2007).
Aspirin for Cardiovascular Disease Prevention
Aspirin Pseudoresistance
Grosser et al. (2013) assessed aspirin resistance and identified a high occurrence of pseudoresistance that is clinically meaningful.
Healthy volunteers (n = 400) were screened for their response to a single dose of 325-mg immediate release or enteric coated aspirin. Response parameters reflected the activity of the molecular target of aspirin, cyclooxygenase-1. Absorption proved very variable and caused up to 49 % apparent resistance to a single dose of enteric coated aspirin but not to immediate release aspirin (0 %). Conclusion: pharmacologic resistance to aspirin is rare; the study failed to identify a single case of true aspirin resistance. Pseudoresistance, reflecting delayed and reduced drug absorption, complicates enteric coated but not immediate release aspirin administration.
The author abandoned the use of enteric coated aspirin in 2009 and instead prescribes soft chewable aspirin 81 mg for CVD prevention.
Sound advice on the use of aspirin therapy for cardiovascular disease prevention is given by the European society of cardiology (Halverson et al. 2014).
Angina RCTs
PCI Versus Optimal Medical Therapy
COURAGE: The Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (trial compared an initial strategy of PCI plus optimal medical therapy with optimal medical therapy alone for patients with stable angina. This RCT studied 2,287 patients who had objective evidence of myocardial ischemia, 1,149 patients to undergo PCI with optimal medical therapy (PCI group), and 1,138 to receive optimal medical therapy alone (medical-therapy group). The primary outcome was death from any cause and nonfatal MI during a median follow-up of 4.6 years). There were 211 primary events in the PCI group and 202 events in the medical-therapy group. The 4.6-years cumulative primary-event rates were 19 % in the PCI group and 18.5 % in the medical-therapy group.
In patients with stable angina, PCI did not reduce the risk of death, MI, or other major cardiovascular events when added to optimal medical therapy (Boden et al. for the COURAGE Trial Research 2007).
During the trial period, 21 % crossed over and received PCI. There was rapidity of improvement in health status in both treatment groups; the majority of patients who received optimal medical therapy alone had improved symptoms within 3 months. This suggests that optimal antianginal medications are underused in practice.
The “take-home” message from the COURAGE trial is to pursue optimal medical therapy initially as it is effective in 75 % and if this is ineffective in 25 %, resort to PCI.
Pooled together the results of 17 randomized trials comparing PCI and medical treatment as strategies in patients with stable angina and no acute coronary syndromes, this meta-analysis concluded that a PCI-based invasive strategy may improve long-term survival in patients with stable coronary artery disease (CAD), and that this justifies a new clinical trial sufficiently powered to evaluate the impact of PCI on long-term mortality.
Based on the strength of available evidence, however, O’Rourke recommends “more aggressive medical therapy for patients with moderate to severe angina, and PCI or CABG for patients whose symptoms persist. Optimal medical therapy is a proven option for chronic stable angina” (O’Rourke 2008).
Carvedilol in Postinfarct Patients
CAPRICORN: In the Carvedilol Postinfarct Survival Controlled Evaluation trial, patients with acute MI and ejection fraction (EF) < 40 % (mean 32.8), 1–21 day prior to randomization, treated with an angiotensin-converting enzyme (ACE) inhibitor, were randomly assigned; the treatment arm received carvedilol 6.25 mg, which was increased progressively to 25 mg twice daily in 74 % of treated patients.
Carvedilol caused a significant 23 % reduction in all-cause mortality in patients with acute MI observed for 2.5 years [mortality 116 (12 %) in the treated versus 151 (15 %) in the placebo arm; p = 0.031] (CAPRICORN Investigators 2001).
The absolute reduction in risk was 2.3 %: 43 patients need to be treated for 1 year to save one life; this reduction is somewhat better than that observed in a meta-analysis of three ACE inhibitor trials, SAVE, AIRE, and TRACE.
Notably, the reduction by carvedilol is in addition to those of ACE inhibitors alone.
Atrial Fibrillation RCTs
Lenient Rate Control
Van Gelder and colleagues (2010) randomly assigned 614 patients with permanent atrial fibrillation to undergo a lenient rate-control strategy (resting heart rate <110/min) or a strict rate-control strategy (resting heart rate <80/min and heart rate during moderate exercise <110 beats/min). The primary outcome was a composite of death from cardiovascular causes, hospitalization for HF, and stroke, and systemic embolism.
Results: The estimated cumulative incidence of the primary outcome at 3 years was 12.9 % in the lenient-control group and 14.9 % in the strict-control group, with an absolute difference with respect to the lenient-control group of –2 % points p < 0.001 for the prespecified noninferiority margin. The frequencies of the components of the primary outcome were similar in the two groups.
More patients in the lenient-control group met the heart-rate target or targets [304 (97.7 %), versus 203 (67 %) in the strict-control group; p < 0.001] with fewer total visits [75 (median, 0), versus 684, p < 0.001].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


