Guides and Wires in Percutaneous Coronary Intervention
Ivan P. Casserly
Irving Franco
In the rapidly changing, innovative world of interventional cardiology, the tendency exists to pay less attention to some of the more mundane, albeit essential, equipment that we use routinely. Guide catheters and guidewires fall into this category. Currently, the skills of appropriate guide catheter or guidewire choice and manipulation are largely taught in a hands-on apprenticelike fashion in the catheterization laboratory. In the near future, medical simulation may play a greater role and even may be required prior to intervention in humans. Devoting a chapter of an interventional textbook to a discussion of guide catheters and guidewires may at first seem like “the intellectualization of a nonintellectual endeavor,” however, intellectual elements are present in this discussion that can be successfully conveyed in a written format that will translate into better practice in the catheterization laboratory.
GUIDES
In the first coronary angioplasty, performed in 1976 by Gruntzig, a guide catheter was used to fulfill the functions of providing a conduit for the delivery of interventional equipment to the coronary ostium and sufficient support for the successful passage of that equipment to the target site in the coronary tree (1,2). These basic functions of a guide catheter remain true today. Early modifications to the original angioplasty technique resulted in the guide catheter performing the secondary ancillary functions of providing assessment of pressure at the guide tip and allowing contrast injection for visualization of the coronary tree during the procedure.
The conduit function of a guide is a straightforward characteristic and is largely dependent on the internal diameter of the guide. In contrast, the support function is significantly more complicated (3). The inherent “passive” support provided by a guide is dependent on the specific construction of the guide catheter wall and the distal preformed shape of the guide. The latter impacts the ability of the guide to achieve coaxial alignment with the coronary ostium, and additionally, to gain added support from either the aortic wall or coronary sinus. “Active” guide support may be achieved by operator-dependent techniques, such as deep-seating the guide beyond the vessel ostium or by “molding” the catheter in the coronary sinus.
Other desirable characteristics of guide catheters provide for added ease of use and safety. These include a soft tip to minimize the risk of ostial trauma, radio-opacity for ease of visualization, the ability to transmit torque applied of the guide hub to the distal tip in a 1:1 ratio, flexibility in tortuous aorta and peripheral vessels, and resistance to kinking in response to guide torque. These characteristics of guides are interrelated and, in some instances, are competitive. For example, increasing the internal diameter of a guide at the expense of wall thickness may enhance the conduit function and reduce the risk of ostial trauma, but may compromise the support function and torque control of the guide. Current guide catheter designs represent a compromise between these competing characteristics. Although there is some variation between manufacturers,
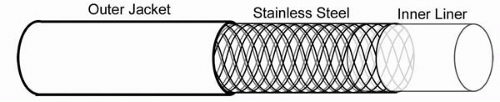
all guides have a basic three-layer construction (Fig. 7.1). The inner liner is made of a lubricious material, typically Teflon or PTFE Teflon, which facilitates the passage of interventional equipment. A middle braided layer of stainless steel or Kevlar contributes to much of the support function, torque control, and kink resistance of the guide. The composition of the outer jacket is highly varied among manufacturers, with polyethylene and polyurethane being the most commonly used components. Both are lubricious and nonthrombogenic and contribute to the overall support function and kink resistance of the guide. Most guides have a multisegment design with variation in the three-layer construction between the guide hub and tip. In general, those segments closest to the hub are modified to provide more support, whereas those closest to the tip are more flexible to facilitate coaxial alignment of the guide and minimize the risk of ostial trauma.
all guides have a basic three-layer construction (Fig. 7.1). The inner liner is made of a lubricious material, typically Teflon or PTFE Teflon, which facilitates the passage of interventional equipment. A middle braided layer of stainless steel or Kevlar contributes to much of the support function, torque control, and kink resistance of the guide. The composition of the outer jacket is highly varied among manufacturers, with polyethylene and polyurethane being the most commonly used components. Both are lubricious and nonthrombogenic and contribute to the overall support function and kink resistance of the guide. Most guides have a multisegment design with variation in the three-layer construction between the guide hub and tip. In general, those segments closest to the hub are modified to provide more support, whereas those closest to the tip are more flexible to facilitate coaxial alignment of the guide and minimize the risk of ostial trauma.
Most coronary interventional procedures are performed using 6, 7, or 8 Fr guide catheters. Our personal bias is to use 8 Fr guides for all but the most straightforward procedures. This reflects a philosophy of relying on the inherent passive support supplied by the guide in an effort to minimize the need for additional guide catheter manipulation and the associated risk of trauma to the coronary ostium and proximal vessel. For operators who prefer 6 Fr guides, more aggressive guide manipulation to achieve added active guide support is required more frequently. Advantages and disadvantages accrue to either approach, and some flexibility based on individual circumstances is warranted. For example, the presence of severe peripheral vascular disease or coronary ostial disease argues in favor of using a smaller guiding catheter. Lesion-related factors, such as heavy calcification, severe proximal tortuosity, total occlusions, and bifurcation lesions, argue in favor of using a larger guide. Finally, one must consider the minimum internal diameter requirement of the interventional equipment one wishes to deliver to the coronary lesion. The constantly evolving array of interventional devices requires that the operator always confirm that the guide size chosen will accommodate a particular piece of equipment.
Most guide catheters are available with side holes just proximal to the guide tip. Some operators use such guides in the presence of coronary or graft ostial disease. The rationale for such guides is that they facilitate the maintenance of coronary perfusion by allowing blood flow through the side hole into the coronary vessel when the presence of ostial disease does not allow blood flow around the guide tip. The major risk is that the pressure transduced from the side holes completely masks the ability to appreciate malalignment of the guide tip with the coronary ostium, thus increasing the risk of dissection. Therefore, our practice is to completely avoid the use of such guides.
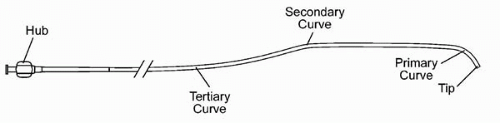
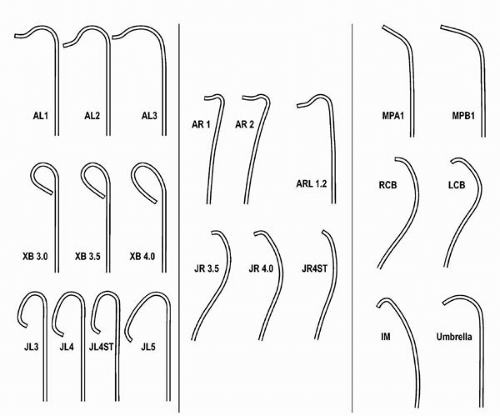
Guides are differentiated by the preformed shape of their terminal portion. All guides have a primary curve located closest to the guide tip. Some guides have additional secondary and/or tertiary curves located in proximal sequence from the primary curve (Fig. 7.2). The requirement for coaxial alignment of the guide with the coronary ostium has resulted in the production of a much larger array of guide shapes, compared with diagnostic catheters (Fig. 7.3). Minor variation often exists between the curves on similarly named guide and diagnostic catheters. For example, the JR guide has a shorter tip and more open primary curve, compared with the JR diagnostic catheter. Additionally, some typical minor variation exists between similarly named guides from different manufacturers.
Our strategy with respect to guide selection for various native and graft coronary intervention is outlined in the following section. It is impossible for an operator to be proficient with the use of every available guide. A pragmatic approach is to restrict one’s primary inventory to a few preferred guides, and to become expert in the nuances
of their use. Using such an approach, most operators develop simple algorithms toward guide selection that involve a relatively small number of guides. Additional guides may be used in exceptional circumstances. The critical element in the success of such a strategy is to develop a comprehensive understanding of factors that influence a successful choice of guide, including the anatomy of the aortic arch and root, the location of the coronary or graft ostium, the orientation of the ostium and proximal vessel segments, the geometry of guide shapes, the typical behavior of guides in response to manipulation by the operator, the nature of the target lesion, and the characteristics of the vessel proximal to the target lesion. Although didactic training can assist in an appreciation of these elements, there can be no substitution for hands-on experience in a large number of cases and supervision by highly experienced teachers.
of their use. Using such an approach, most operators develop simple algorithms toward guide selection that involve a relatively small number of guides. Additional guides may be used in exceptional circumstances. The critical element in the success of such a strategy is to develop a comprehensive understanding of factors that influence a successful choice of guide, including the anatomy of the aortic arch and root, the location of the coronary or graft ostium, the orientation of the ostium and proximal vessel segments, the geometry of guide shapes, the typical behavior of guides in response to manipulation by the operator, the nature of the target lesion, and the characteristics of the vessel proximal to the target lesion. Although didactic training can assist in an appreciation of these elements, there can be no substitution for hands-on experience in a large number of cases and supervision by highly experienced teachers.
Left Coronary Artery
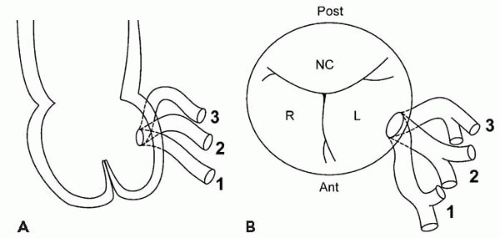
In selecting an appropriate guide for left coronary artery (LCA) intervention, the location of the LCA ostium and its orientation with respect to the aortic sinus must be appreciated (Fig. 7.4). The LCA typically arises orthogonally from the middle portion of the left anterior sinus of Valsalva, just inferior to the sinotubular junction (4). In less than 1% of cases, a separate origin of the left anterior descending and circumflex arteries (LCX) is present, with the circumflex ostium lying posterior to the LAD ostium. The orientation of the left main coronary artery with respect to the coronary sinus may vary from its typical orthogonal plane in both a superior/inferior and anterior/posterior direction.
The workhorse LCA guide in our laboratory is the Extra Backup XB 3.5 guiding catheter. Engagement with this guide is usually straightforward. The guide is advanced over a guidewire with its tip positioned in the left coronary sinus. Removal of the guidewire results in upward movement of the tip of the guide toward the LCA ostium. Clockwise and counterclockwise rotation of the guide will result in anterior and posterior movement of the tip of the guide toward the ostium, which is best determined in the RAO projection. Using this guide, the heel of the guide (i.e., the primary curve) rests against the contralateral aortic wall and, in most cases, the noncoronary sinus. Therefore, larger (XB 4.0, XB 5.0) and smaller (XB 3.0) guides should be used in individuals with larger and smaller aortic root and aortic sinus sizes, respectively. For individuals with superiorly oriented left main coronary arteries, the smaller XB guide also provides more coaxial alignment. The reverse holds true for inferiorly oriented left main coronary arteries.
The Amplatz Left (AL) guide remains a popular choice in our laboratory and is particularly useful for cases in which extra support is required. Correct manipulation of this guide is imperative due to the risk of ostial trauma, thus making careful instruction of trainees by experienced operators essential. The guide is delivered to the aortic root over a guidewire. In most instances, the tip of the AL guide initially will find the right coronary sinus. The guide is withdrawn, and clockwise rotation usually will result in the tip finding the left coronary sinus. Several attempts with to-and-fro movements of the guide in slightly different orientations may be required to achieve this. With the tip in the left coronary sinus, the catheter is rotated counter-clockwise (bringing the tip more posterior) and pushed forward, resulting in the catheter tip rising in the aortic sinus toward the coronary ostium. As with the XB guide, the RAO projection then is used to judge the anterior-posterior plane of the ostium, and the guide rotated clockwise and counterclockwise to move the guide anteriorly and posteriorly, respectively. The heel of this guide (i.e., the secondary curve) typically rests against the non-coronary sinus. One of the myths surrounding the use of the AL guide relates to the appropriate method of disengagement. We observe disengagement of the AL guide in the LAO projection and, in >95% of cases, careful withdrawal of the guide results in successful disengagement without further intubation of the left main coronary artery.
In our experience, the AL 2 guide is an appropriate size for most male patients, while the AL 0.5 or AL 0.75 guide is an appropriate size for most female patients. The AL 0.5 guide must be specifically requested from the manufacturer, because it is not routinely available. AL 3 and AL 4 guides are available for individuals with larger aortic roots and sinuses, or with a superior location of the left coronary ostium. Rarely, when an AL guide does not engage the left coronary ostium from its typical location in the left coronary sinus, a larger AL guide with its secondary curve located in the right coronary sinus may be successful. The AL guide is ideally suited for left main coronary arteries with a superior orientation. Most authors highlight the advantage of the AL guide in preferentially aligning with the circumflex artery
due to the inferior orientation of the terminal portion of this guide tip. Careful advancement of the guide, however, will remove this inferior orientation of the tip and allow similar success with left anterior descending interventions.
due to the inferior orientation of the terminal portion of this guide tip. Careful advancement of the guide, however, will remove this inferior orientation of the tip and allow similar success with left anterior descending interventions.
We generally reserve Judkins Left (JL) guides for the most straightforward left coronary interventions in which minimal support is anticipated (e.g., proximal LAD lesion in noncalcified vessel). The failure to supply adequate support for most interventions is the major downfall of this guide. The same considerations with regard to guide catheter size and aortic root size described for the XB guide also apply to the JL guide. In rare circumstances, a JL guide will be the only guide that selectively engages a superiorly oriented left main coronary artery. Short-tipped JL guides also are useful when treating left main coronary artery stenoses, particularly ostial lesions, where the lack of robust support is often an advantage in minimizing guide tip trauma to the ostium.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree