Fig. 12.1
Three in situ arterial grafts: left IMA, right IMA, and right GEA
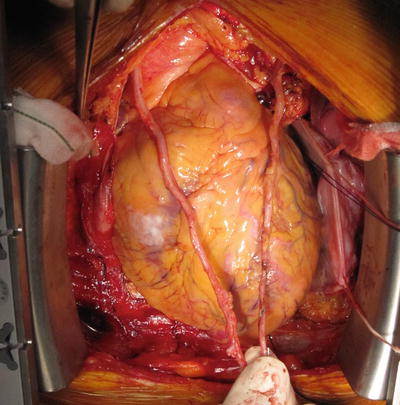
Fig. 12.2
Two mammary arteries: the skeletonized RIMA is long enough to reach the distal LAD
Some study showed good results of composite Y- or T-graft of the free RITA connected to the in situ LITA. A composite graft can increase the number of anastomoses and may cause damage to the donor artery and undesirable distribution of graft flow to the larger area. Although the results of composite graft are acceptable, almost all reports showed better patency rate and long-term outcomes of the in situ graft than the composite graft. Thus, the ITAs should be used as the in situ graft whenever technically possible.
12.1.3 Right Gastroepiploic Artery
The history of using the right gastroepiploic artery (GEA) in coronary revascularization started in the 1960s, when Bailey reported the Vineberg implantation of the GEA into the posterior area of the heart. Since direct coronary artery bypass procedures became the standard revascularization method, the GEA to coronary artery anastomosis has been reported by Pym [10] and Suma [11] in 1987.
The GEA is the main branch of the gastroduodenal artery which arises from the right hepatic artery. After the GEA gives off branches to the pancreas, duodenum, and the pylorus, it runs along the greater curvature of the stomach. In contrast to the IMA, the GEA has fewer elastic lamellae in its media and is classified as a muscular artery [12].
The GEA provides at least 20 cm of useable length which can reach all areas of the heart. Skeletonization using ultrasonic scalpel makes the GEA longer and wider, so that it can be anastomosed at a more proximal position than the pedicled GEA. In 1998, the use of skeletonized GEA has been reported for coronary bypass conduit [13]. However, the skeletonization technique is cumbersome and time-consuming with ordinary methods using electrocautery, scissors, and hemoclips. In 2001, we developed a simple and safe technique for harvesting skeletonized GEA using an ultrasonic scalpel (Harmonic Scalpel, Ethicon Endo-Surgery, Cincinnati, Ohio) [14]. The size of the skeletonized GEA has surprised us since we have been using the technique described above. It has ranged from 2.5 to 6.5 mm in internal diameter at the site of the distal anastomosis (Fig. 12.3). This is quite different from the size of conventionally prepared GEA with surrounding tissues, which was reported to be 1.25–2.5 mm in earlier work. The difference implies that skeletonization may play an important role in dilating GEA maximally. I believe it is most important that we prepare each GEA conduit at its maximal dilatation prior to anastomosis. What we have found since we started the skeletonization technique is that the first appearance of GEA does not predict the potential size of the artery. The vascular tonus of GEA can vary significantly during an operation. Care should be taken not to underestimate the size of GEA at the beginning of operation. In no case the GEA was too narrow for use after the proper skeletonization technique. The suitable targets of the in situ GEA are the distal right coronary artery or the distal circumflex artery. It is very feasible to perform sequential grafting using skeletonized GEA for its sufficient length and diameter.
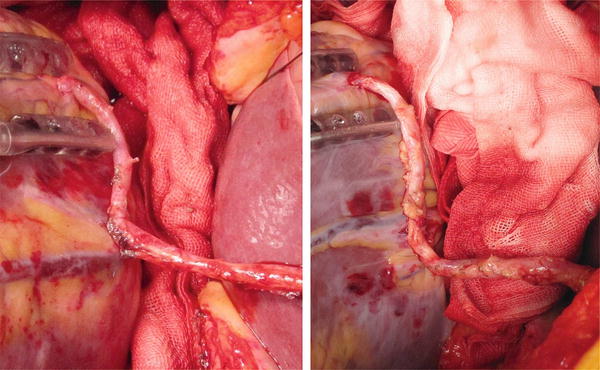

Fig. 12.3
Skeletonized GEA has sufficient length and adequate large diameter that make easy sequential grafting
The early functional patency rate of the skeletonized GEA is reported to be better than that of the nonskeletonized GEA. Kim and coworkers [15] evaluated the early and 1-year postoperative results of grafting the skeletonized GEA to the RCA and found an excellent early patency rate of 98.3 % and 1-year patency rate of 92.0 %.
There are little in the literature of mid- and long-term patency rate of the GEA. Suma and coworkers [12] reported their 20 years’ experience of using GEA graft that the cumulative patency rate of the GEA was 97.1 % at 1 month, 92.3 % at 1 year, 85.5 % at 5 years, and 66.5 % at 10 years, respectively. Of them, in 172 skeletonized GEA graft with 233 distal anastomoses, the patency rate at 1 and 4 years after surgery was 92.9 % and 86.4 %, respectively. Ali and colleague [16] focused and evaluated the skeletonized GEA patency rate collecting the data from 11 clinical papers. Overall patency rates were 97.7 % within three months, 92.4 % at a mean of ~1 year, 91.5 % at a mean of 2 year, and 86.4 % at 4 years. In 2013, we presented our single-center report that the cumulative patency rate of the skeletonized GEA was 97.8 % at 30 days, 96.7 % at one year, 96.0 % at 3 years, 94.7 % at 5 years, and 90.2 % at 8 years after surgery that is superior to that for pedicled GEA or saphenous vein graft [17] (Fig. 12.4). It can be said that skeletonized GEA is a reliable conduit and assumes a large role in all arterial OPCAB strategy. However, the GEA has not necessarily gained acceptance among cardiovascular surgeons worldwide. Reasons for its less frequent use include concerns about insufficient flow capacity and vasospasm, the need to open the abdomen, and competitive flow causing graft failure. We need to open the abdomen when using the GEA; however, the skin incision extends only 3–4 cm. It is well known that flow competition may occur when the GEA is anastomosed to coronary artery with low-grade stenosis. As a general rule, we use the GEA only when the coronary artery stenosis is severe. We think that late graft occlusion may occur in association with flow competition. In our study, we discovered through multivariate analysis that low-grade target vessel stenosis was a strong risk factor for late GEA occlusion. We therefore recommend again that the GEA should in principle be used for more severe stenosis (>75 %).
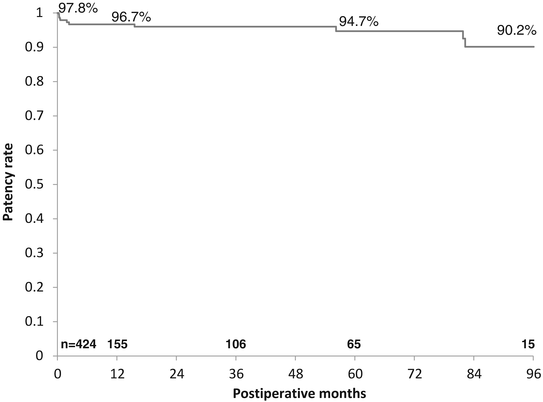

Fig. 12.4
Cumulative patency rate of the GEA graft by the Kaplan-Meier method
Surgeons have not necessarily achieved great understanding of the potential ability of the GEA that has been undervalued. We think that the GEA will play a critical role in an area of OPCAB procedure with in situ all arterial grafting and surgeons should discuss and grasp the detail of the potential power of the GEA.
12.1.4 Radial Artery
The radial artery is frequently used as second graft of choice after the ITAs in CABG. Carpentier [18] et al. firstly reported the use of radial artery in CABG; however, it was soon abandoned because of disappointing early angiographic outcomes. After improvement of harvesting technique, management of pharmacologic dilatation, and the use of postoperative calcium channel blocker, the radial artery was reviewed according to encouraging reports in the 1990s.
The radial artery is a very convenient conduit that has several advantages including the ease of harvesting, sufficient length for grafting to almost any coronary artery territory, and suitable caliber for matching coronary artery. The radial artery is the muscular artery and has more spastic character than other arterial conduits. To prevent the spasm of the radial artery, intravenous nitrates or calcium channel blockers should be used intraoperatively as well as postoperatively until the patient can take oral medications. The endothelium of the radial artery plays an important role for preventing vasospasm. To preserve the endothelial function, the surgeon should take caution not to inflict excessive manipulation on the radial artery and not to mechanically dilate it during harvesting.
The radial artery is often used as composite graft connected to the ITA in end-to-side fashion. Effort to achieve aorta no-touch strategy in OPCAB procedure has led to the increased use of the RA as a composite bypass graft. Although the patency rate of radial artery composite graft is acceptable, almost all studies showed the superiority of direct aortocoronary bypass to composite graft. Therefore, when the RA composite grafting is performed, careful consideration of undesirable distribution of the blood flow due to unbalanced vascular area is needed.
The early (~12 months) patency rate of the radial artery is very favorable that is expected more than 90 %. Recently, the long-term (5years~) patency rate has been reported to be between 70 and 98 %. The patency differences of the radial artery are more significantly influenced by target coronary territory and target vessel stenosis than other conduits. It is said that if the proximal stenosis is 90 % or more, radial artery patency between ITA-RA composite graft and direct aortocoronary bypass was similar. Therefore, when the proximal native stenosis is milder, the radial artery should be used as direct aortocoronary anastomosis.
In the current circumstance of aorta no-touch OPCAB, the radial artery is a supplemental conduit used in cases which the bilateral ITAs are not available or complete reconstruction of left side coronary area cannot be achieved with bilateral ITAs alone.
12.1.5 Saphenous Vein Graft
The saphenous vein is the most popular and easily handled for graft to the RCA or the CX, and the relevant long-term clinical outcome, flow capacity, patency, and long-term complications are well known. As the advantage of the arterial conduit has become evident, the use of the SVGs has decreased. Now, in an aorta no-touch arterial OPCAB era, the SVGs are used in limited cases, including nonavailable GEA, RCA target with milder proximal stenosis, hemodialysis or renal failure patient in that the RA should not be used, and emergency with hemodynamic instability. The reasons for less use of the SVGs are as follows: wound complication such as edema, cellulitis, and delayed healing causing patients’ discomfort; need for aorta manipulation during proximal anastomosis; and so-called graft disease that worsens long-term outcome. The SVG may developed intimal hyperplasia in early phase within 1 year, furthermore, atherosclerotic change in long term (>5years) that related to graft occlusion more frequently than arterial conduits. The patency rate of the SVG may increasingly deteriorate with time, as we know, that is 50–60 % at 10 years after CABG. Endoscopic vein harvesting technique has been developed for the purpose to reduce the clinical complications including wound infection, leg edema, pain, and prolonged hospital stay. Although the endoscopic vein harvesting technique has been widely spread among surgeon, the clinical result is disappointing. Lopes et al. reported the clinical outcome comparing endoscopic harvesting to open harvesting and concluded that endoscopic vein-graft harvesting is independently associated with vein-graft failure and adverse clinical outcomes. Thus, the endoscopic vein harvesting technique should be used only in selected patient. The damage of the endothelium of the SVGs often leads to platelet aggregation that causes intimal hyperplasia resulting to graft occlusion. Recently, no-touch technique of the SVG harvesting is recommended that provides a pedicled graft. It preserves normal intact adventitia, vasa vasorum, medial blood flow, and endothelial function. The perivascular fat protects the vein against arterial hemodynamics and kinking result in preventing future atherosclerosis. Early SVG failure is associated with distention-induced endothelial denudation. Superior long-term patency rate of pedicled SVG is demonstrated in recent review. In either approach of endoscopic or open harvesting, a careful handling of the SVG during harvesting is important not to damage the endothelium.
12.2 Aorta No-Touch Technique with All Arterial Grafting
12.2.1 An Advantage of Aorta No-Touch Technique
The incidence of stroke in patients undergoing routine CABG is reported to be 1–3 %, most commonly as a result of ascending aortic embolic phenomena. Various studies have proven embolic showers using transcranial Doppler during cannulation, clamping, or declamping maneuvers and especially in association with the release of the aortic cross-clamp. The best strategy to reduce stroke risk appears to be to completely avoid handling the aorta, the so-called aorta no-touch technique. An effort to reduce stroke during CABG has led to widespread use of aorta no-touch strategy with off-pump technique. The main indication for no-touch aorta OPCAB operation is patients who have a severely atherosclerotic or porcelain ascending aorta.
Kapetanakis and associates [19] reported that stroke rate with on-pump CABG was 1.5 times (2.2 % versus 1.6 %) that with off-pump CABG with partial aortic clamping and three times (2.2 % versus 0.8 %) that in the no-touch aorta OPCAB group. Kim and associates [20] reported a lower incidence of perioperative stroke with the no-touch aorta OPCAB technique, but similar stroke rates between OPCAB with aortic manipulation and conventional on-pump CABG. Misfeld and coworkers [21] presented that 81 of 5,779 (1.4 %) patients in the OPCAB group with aortic manipulation had strokes, compared with 29 of 5,619 (0.5 %) patients in the aortic no-touch group. Lev-Ran and coworkers [22] reported one neurologic event among 429 consecutive patients (0.2 %) in the no-touch OPCAB group, which compared favorably with a stroke rate of 2.2 % observed in the side-clamp OPCAB group. On the other hand, complete avoidance of aortic manipulation preserves the aortic fat pad containing neurogenic tissue and thereby may avoid an autonomic imbalance and further decrease the incidence of atrial fibrillation. Kim and coworkers [23] demonstrated that OPCAB with complete avoidance of aortic manipulation significantly reduced the incidence of postoperative atrial fibrillation compared with conventional CABG (11.4 % versus 21.1 %).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


