Fig. 15.1
Gene therapy clinical trial data. (a) Relative distribution of clinical trials by disease. (b) Relative distribution of cardiovascular gene therapy clinical trials by vector used
15.2 Gene Therapy Strategy
Two basic types of gene therapy strategies are currently employed in cardiovascular disease.
The first strategy is the exogenous overexpression of a target gene, aiming to increase the activity of a gene whose endogenous function may be impaired or downregulated as a result of mutation or a pathological process. In this case, cDNA encoding the deficient gene is delivered to the nuclei and the replaced gene product is expressed and interacts with a defined cell mechanism. The goal is to restore normal function or reverse disease progression.
The second strategy is the inactivation or silencing of target genes exhibiting maladaptive activity. Potential approaches in this strategy include (i) expression of a peptide or protein inhibitor, (ii) the use of truncated proteins to express only the part of the protein aimed at direct inhibition of enzyme function or disruption of protein-protein interactions, and (iii) the use of RNA interference technology [5]. This strategy can be performed at the transcriptional level (antisense oligonucleotides) or at the posttranscriptional level (ribozymes and small interfering RNA).
Double-stranded oligonucleotides (decoy) have been used to inhibit transcription factors involved in the activation of pathogenic genes [6].
Short single-stranded deoxyoligonucleotides (antisense oligonucleotides) bind to the target gene mRNA and prevent it from being translated.
Small interfering RNAs (siRNAs) are short RNAs that knock down the endogenous activity of a pathogenic gene by induction of sequence-specific gene modification.
Proof of principle of efficient RNA interference using adenovirus-based vector was demonstrated in the phenotyping of cardiac myocytes [7].
Ribozymes are used to degrade target mRNA transcripts copied from the gene. Selective blockade with ribozyme oligonucleotides in a rat model resulted in inhibition of neointimal formation after vascular injury [8].
15.2.1 Ex Vivo and In Vivo Gene Therapy
Ex vivo gene therapy involves the harvest of cells from a patient followed by therapeutic gene replacement or addition into the target cell genome via vector. After this transduction, the cells are returned to the patient to exert their therapeutic effect. As an example, a study on VEGF was performed in a murine model. After 7 days in culture (ex vivo), endothelial progenitor cells (EPCs) were transduced with an adenovirus encoding the VEGF 164 gene. This manipulation augmented EPC proliferative activity and enhanced adhesion and incorporation of EPCs into quiescent and activated endothelial cells. After that, gene-modified EPCs were administered to mice with hind limb ischemia. Neovascularization and blood flow recovery were both improved, and limb necrosis was reduced by 63.7 % compared to control animals [9]. This ex vivo technique can allow for increased transgene expression, but can only be applied to certain cell types. Conversely, in vivo gene therapy involves the introduction of a virus carrying the genetic material directly into body tissue.
15.3 Main Prerequisites for Successful Cardiovascular Gene Therapy
Achieving specificity and efficiency of cardiovascular gene therapy requires the application and interaction of several essential factors:
(i)
Selection of appropriate transfer vector and promoter
(ii)
Development of targeted route and technique of gene delivery
(iii)
Validation of the correct transgene molecular targets
Genetic material must be transferred into cells and expressed either at a constant level with insertion of the DNA into the cell genome or on a temporary basis with preservation of the DNA in an episomal state. Only use of special vehicles called vectors results in sufficient transport of genetic information into a cell. Vectors can be divided into viral and nonviral delivery systems. The optimal vector should be safe, have the ability to be transduced in vivo or ex vivo with reinfusion to the patient, restrict expression to the desired tissue, provide the desired longevity of expression, have sufficient capacity for the genetic material to be transferred, and minimize the risk of an immune response.
15.3.1 Nonviral Vectors
Nonviral gene therapy targeting the cardiovascular system began to develop before viral vectors and is still the most prevalent approach in clinical trials (Fig. 15.1b). The basic advantages of nonviral vectors include a relative lack of inflammatory and immune responses (allowing gene reintroduction) as well as low toxicity and the absence of a potential for mutagenesis, removing the safety concerns characteristic of viruses. Moreover, there is no limiting size for transgene and both ease of production and stability over time [10]. The materials used in nonviral cardiovascular applications include plasmid DNA (pDNA) and small nucleic acids (antisense oligonucleotides or small interfering RNAs). pDNA is a double-stranded DNA encoding the gene of interest. The most significant limitation of nonviral vectors is a low transfer efficiency resulting in poor transgene expression. To improve this, many investigators have begun using chemical-based vectors such as cationic lipids and cationic polymers which promote cell uptake and trafficking and protect DNA from intracellular degradation. Left ventricular injection of pDNA in vivo was first performed about 25 years ago. Marker gene expression was observed in myocytes 3–4 weeks after delivery [11]. By 1998, a phase I clinical trial demonstrated the safety and efficiency of gene transfer of pDNA encoding vascular endothelial growth factor (VEGF) in patients with thromboangiitis obliterans (Buerger’s disease) [12]. The same year, naked pDNA encoding VEGF was used as a sole therapy for patients with symptomatic ischemic heart disease (IHD). All patients had reduction in angina and improvement of myocardial perfusion on coronary angiography [13]. In another clinical trial, 28 patients with coronary heart disease (angina class II–III) received catheter-based intracoronary delivery of VEGF-plasmid liposome. This study demonstrated the safety and feasibility of liposome-mediated gene transfer [14].
15.3.2 Viral Vectors
Genetic engineering of vector from virus requires that coding genes and cis-acting sequences be separated into distinct nucleic acid molecules to prevent their reconstitution by recombination into productive viral particles. Linking of the viral cis-acting sequences (noncoding DNA regulating gene transcription) to the therapeutic gene produces replication-deficient particles able to transfer new genetic information [15]. The majority of viral vectors used for cardiovascular applications were derived from human pathogens from which essential genes have been deleted. Several viral vectors have been explored successfully for cardiovascular gene transfer because of their various advantages (Table 15.1).
Table 15.1
Available vector systems for cardiovascular gene therapy
I. Nonviral | |
Advantages | Limitations |
(a) Naked pDNA | |
Simple methodology | Low transduction efficiency |
No limit to transgene size | Transient gene expression |
Little immunogenicity and oncogenicity | |
(b) Antisense oligonucleotides | |
Easy to produce | Limited efficiency |
No immune response | Degradation by nucleases |
No integration in host cell genome | Transient effect |
II. Viral | |
Advantages | Limitations |
(a) Adenovirus | |
High transgene capacity | Immune-inflammatory response |
High transduction efficiency | Short-term expression |
Transfer to cardiac and vascular cells | |
(b) Adeno–associated virus | |
Long-term gene expression | Limited transgene capacity |
Low immunogenicity | Difficult to produce in large quantities |
High tropism to cardiac and vascular cells | |
(c) Lentivirus | |
Long-term expression | Integration into the host cell genome |
Low immune response | Limited cardiovascular tropism |
Risk of oncogenicity |
15.3.2.1 Lentiviral Vectors
These vectors are derived from primate and nonprimate immunodeficiency viruses. Expression and therapy are sustained and induce nonsignificant immune response. Maintenance of uncompromised cellular function and gene transfer to nondividing cells make these vectors attractive for a wide range of disease targets including cardiovascular diseases. It was shown that lentivirus-based vectors can effectively transduce well-differentiated cardiac myocytes and fibroblasts [16]. Third-generation lentiviruses with a majority of their native genome deleted can transduce human saphenous vein endothelial cells and smooth muscle cells (SMC) better than adeno-associated virus (AAV) serotypes [17]. The major limitation of lentiviral vectors is the risk of mutagenesis and oncogenesis.
15.3.3 Adenoviral Vectors
Adenoviruses contain a double-stranded DNA genome which remains episomal after introduction. Although this type of vector has historically been the most commonly used for preclinical and clinical studies in cardiovascular gene therapy, the pendulum is swinging toward the use of adeno-associated virus for many of these applications. The prevalent use of this vector is due to high expression kinetics, large cloning capacity, broad target cell tropism, efficient levels of transgene expression, and ease of high-titer manufacturing. Porcine hearts infected with an adenovirus vector containing the β-galactosidase (β-gal) gene showed significantly increased β-gal enzymatic activity compared to hearts injected with β-gal plasmid, with the efficiency of adenovirus-mediated gene transfer 140,000 times superior to plasmid DNA injection [18]. Clinical trials using recombinant adenoviral vectors to deliver angiogenic growth factors demonstrated therapeutic benefit [19]. However, adenoviral vector disadvantages such as innate and adaptive immune responses, transient gene expression, and a propensity to trigger inflammatory and toxic reactions in the host undoubtedly restrict its use.
15.3.3.1 Adeno-associated Viral Vectors
Lack of human pathology, low immunogenicity, strong tissue tropism for the heart and vessels, and efficient transduction of cardiomyocytes and SMC make this vector very attractive for cardiovascular applications. AAV can transfer a single-stranded DNA only about 20–25 nm (4.7 kb) in size. At least 11 AAV serotypes have already been described and a much larger number can be engineered through recombination of existing AAV viral capsid sequences [20]. The development of new AAV capsids significantly promoted AAV gene transfer technology in the last decade. Efficient transduction and persistent transgene expression in cardiac tissue were demonstrated in different animal models [21]. The ability of various AAV serotypes to transduce vascular cells in vitro and in vivo has also been proven [22]. Major concerns about AAV vector are its small packaging capacity, difficult production of high-titer vector stocks, and presence of preexistent neutralizing antibodies in at least 50 % of the human population.
15.4 Gene Delivery Techniques for Cardiovascular Applications
A great number of gene delivery techniques have been identified since the onset of cardiovascular gene therapy (Fig. 15.2). Two major conclusions should be drawn from the data: the route of gene delivery is no less important than the vector system, and gene delivery should be organ targeted with minimal or optimally zero collateral expression.
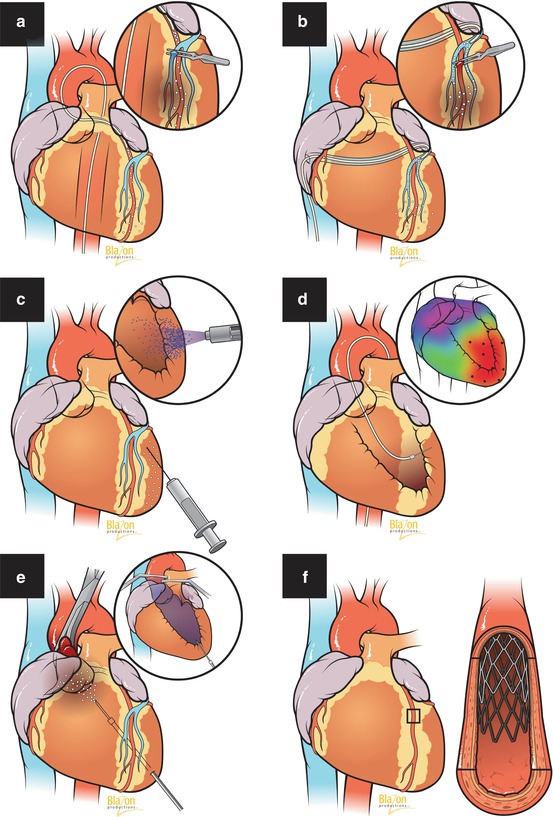

Fig. 15.2
Gene therapy delivery methods. (a) Antegrade intracoronary delivery (inset: with venous blockade). (b) Retrograde transcoronary sinus (inset: with arterial occlusion). (c) Intramyocardial injection, epicardial approach (inset: liquid jet injection). (d) Intramyocardial, endocardial approach (inset: image-guided delivery). (e) Intracavitary injection to left ventricle with aortic occlusion (inset: with aortic and pulmonary artery occlusion). (f) Transvascular intracoronary wall delivery via gene-eluting stent (inset: stent within artery)
15.4.1 Transvascular Route
Intravenous administration is the least invasive and simplest route for gene transfer. It finds its application in the treatment of diseases such as systemic hypertension and hyperlipidemia. However, in peripheral arterial disease as well as acquired and congenital cardiac disorders, this technique is ineffective due to first-pass pulmonary and hepatic uptake of vector and systemic dilution in blood circulation. Efficacy of antegrade intracoronary administration is much better, although it cannot escape systemic leakage. In an effort to achieve increased transduction, researchers began to use concomitant coronary venous blockade [23], transient coronary occlusion [24], and increased perfusion pressure [25]. Retrograde gene delivery through the coronary sinus enhanced expression due to a more than tenfold increase in coronary passage time [26] and an increase in venous capillary filtration rate [27]. Another significant advancement was the creation of a closed–loop recirculatory system which allowed separate heart and systemic circulation [28, 29]. Gene-eluting stents for localized transvascular wall delivery in cardiovascular pathology represent a new attractive alternative to standard angioplasty and vascular interventions. However, this method still needs additional research to assess its transduction efficiency.
15.4.2 Direct Intramyocardial Delivery Using Mechanical and Physical Approaches
Unlike the transvascular route, during direct gene delivery, transgene enters the extracellular matrix and somatic cells, bypassing the blood compartment which includes plasma proteins, blood cells, and neutralizing antibodies which substantially inactivate the vector. Moreover, this method allows for the application of high concentrations of transgene directly at the target site. This approach has been successfully applied in animal models of ischemia and cardiac arrhythmias as well as several clinical trials to induce angiogenesis [30]. Relatively low transduction efficiency led to use of image guidance devices such as the Noga system and a variety of physical and mechanical approaches enhancing cell membrane permeability for gene transfer. The most commonly used are electroporation which involves high-intensity electric pulses, sonoporation which involves attachment of genes to gas-filled microbubbles which are destroyed by ultrasound after injection, use of energy sources such as laser to induce tiny holes in cell membranes, and transfer of gene nanoparticles under the influence of a magnetic field. Liquid jet injection, gene gun particle bombardment, and microinjection are additional mechanical methods which have found application in direct gene delivery for cardiovascular disease.
15.5 Molecular Targets
Identification of potential targets in cardiovascular disease is a very complex and laborious process closely associated with the development of basic science in molecular biology and genetic engineering. For each new target, it is necessary to determine signaling pathways, cell membrane receptors, transcription factors, intracellular trafficking, and many other items that make it possible to influence them through gene therapy (Table 15.2).
Table 15.2
Gene therapy targets in cardiovascular disease
Cardiovascular diseases | Aims | Target genes |
|---|---|---|
Hyperlipidemia | 1. Reduction of LDL cholesterol | ApoB, apoE, apoA-1, eNOS, LDL receptor, NF-κB, VLDL receptors, decoy MSR, TNF-α, TIMPs |
2. Target atherosclerotic lesions | ||
3. Modulation of MSR activity | ||
Systemic hypertension | 1. Inhibition of genes involved in BP elevation | Kallikrein, adrenomedullin, eNOS atrial natriuretic peptide, angiotensinogen, β-adrenergic receptor, ACE, angiotensin receptor |
2. Inhibition of vasoconstriction-promoting genes | ||
Pulmonary hypertension | 1. Suppression of pulmonary SMC proliferation and differentiation | BMPR2, eNOS, CGRP, prostacyclin synthase, adrenomedullin, VEGF, HGF |
2. Inhibition of pulmonary vascular remodeling | ||
Heart failure | 1. Enhancing excitation-contraction coupling | SERCA2a, S100A1, phospholamban, PP-1, βARs, βARKct, adenylyl cyclase, Bcl-2, P13, Akt, HSP, TNF-α, HO-1, SDF, angiotensin II, endothelin 1, TIMP-1, parvalbumin, TGF-β system |
2. Reduction of adverse remodeling | ||
3. Inhibition of apoptosis | ||
4. Abrogation of fibrosis | ||
5. Cytoprotection, stem cell repair | ||
Cardiac arrhythmias | 1. Heart rate control | Gαi2, KCNE3, connexin-43, KiR(2.1b), TGF-β, βARs, HCN2, Kv1.5 |
2. Biological pacemaker function | ||
3. Repolarization and reduction of QT interval | ||
4. Modulation of cardiac conduction | ||
Peripheral arterial disease | 1. Therapeutic angiogenesis | VEGF, HGF, FGF, HIF-1α, Del-1, PDECGF, eNOS, TIMPs, HO-1, prostacyclin, SCDF-1α, sonic hedgehog, netrin, thrombopoietin, C-type natriuretic peptide, COX, Ras, Fas ligand, β-interferon, βAR kinase |
2. Stabilizing plaque and diminishing risk of rupture | ||
3. Prevention of thrombosis | ||
4. Limit restenosis after angioplasty | ||
5. Hindering intimal hyperplasia in vein grafts |
15.5.1 Coronary Heart Disease (CHD)
CHD alone causes one of every six deaths in the United States. In 2014, an estimated 620,000 Americans will have a new coronary attack (defined as first hospitalized myocardial infarction or CHD death) and 295,000 will have a recurrent attack [31]. The failure rate of interventional coronary revascularization as a result of restenosis, multiple stenotic lesions, or suboptimal anatomy remains relatively high. Therefore, the possibility that a one-time delivery of transgene to myocardium may induce global therapeutic angiogenesis or improve contractile function appears very attractive. For CHD, the current genetic targets are varied (Fig. 15.3). It is quite likely that in the future, gene products must act on multiple molecular pathways or supplement existing pharmacotherapy of revascularization procedures.
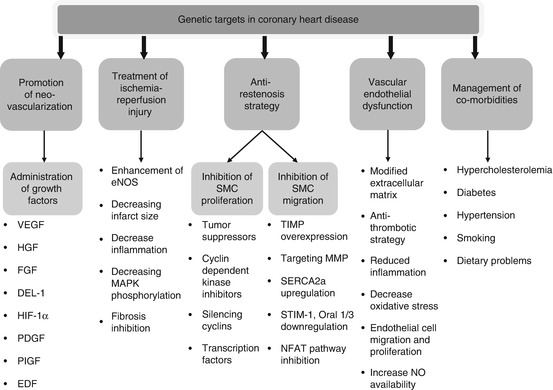

Fig. 15.3
Genetic targets in coronary heart disease. VEGF vascular endothelial growth factor, HGF hepatocyte growth factor, FGF fibroblast growth factor, DEL–1 developmental endothelial locus-1, HIF–1 hypoxia-inducible factor-1, PDGF platelet-derived growth factor, PIGF placental growth factor, EDF erythroid differentiation factor, eNOS endothelial nitric oxide synthase, MAPK mitogen-activated protein kinase, SMCs smooth muscle cells, TIMPs tissue inhibitor metalloproteinases, MMPs matrix metalloproteinases, SERCA2a sarcoendoplasmic reticulum calcium adenosine triphosphatase isoform 2a, SIM–1 stromal interaction molecule-1, Oral 1/3 ORAI calcium release-activated calcium modulator 1/3, NFAT nuclear factor of activated T cells, NO nitric oxide
A vast amount of preclinical and clinical research including phase I–II/III trials has been published regarding angiogenesis [32]. All studies demonstrated excellent safety and feasibility of using recombinant growth factors in CHD [33]. Preclinical gene therapy studies with vascular endothelial growth factor (VEGF) in various animal models of myocardial ischemia have demonstrated improvement in contractility and reduction of both infarct size and peri-infarct fibrosis [34, 35]. Although there was no clear demonstration of a clinical benefit in patients, important factors affecting efficient gene transfer in CHD were identified [36]. The exogenous administration of angiogenic factors including VEGF, hepatocyte growth factor, fibroblast growth factor (FGF), hypoxia-inducible factor-1α (HIF), angiopoietin-1, and insulin-like growth factor is a reasonable approach for therapeutic neovascularization. A phase II KAT trial provided evidence that VEGF-165 gene therapy during percutaneous coronary intervention increased myocardial perfusion [14]. The AGENT study with symptomatic CAD showed that after adenovirus-mediated FGF gene transfer, ischemic defect decreased in patients who were not candidates for revascularization [37]. In the REVASC clinical trial, adenovirus containing VEGF-121 was delivered by direct intramyocardial injection. Administration of VEGF121 resulted in objective improvement in exercise-induced myocardial ischemia [38]. A multicenter, randomized, double-blind, placebo-controlled clinical trial using HIF demonstrated improved perfusion by positron emission tomography analysis [39]. It is not surprising that animal experiments on healthy subjects without comorbidities using recombinant growth factors for angiogenesis were very promising yet the clinical studies demonstrated limited benefits. Furthermore, most of the clinical trials in CHD involved the use of gene therapy for “no-option” patients (patients whose cardiovascular health is poor enough to preclude any other treatments). With these conditions limiting the effectiveness of gene therapy, a single delivery may not cause measurable improvement. Thus, it is very important to choose patients who may respond to gene therapy treatment and to standardize the stage of disease, pharmacological treatment, angiographic findings, and comorbidities.
15.5.2 Hyperlipidemia and Atherosclerosis
Many patients with dyslipidemia cannot achieve optimal cholesterol levels with existing pharmacological therapies [40] (Chap. 28). Therefore, hypercholesterolemia is a promising target for gene therapy. Familial hypercholesterolemia (FH) is an inherited monogenetic disorder caused by low-density lipoprotein (LDL) receptor deficiency. A considerable number of proof-of-principle research studies have been performed in animal models of homozygous FH [41]. The first pilot study of liver-directed gene therapy in patients with FH demonstrated significant and prolonged reductions in LDL cholesterol [42]. In general, gene therapy approaches for atherosclerosis are progressing in two directions: to decrease cholesterol levels in the blood through liver-directed molecular therapy and to target atherosclerotic lesion directly or atherosclerosis-related vascular complications [43]. Apolipoprotein B100 (ApoB) is the main form of LDL and therefore a major target for gene therapy. Blockade of serum ApoB mediated by short interfering RNAs (siRNA) induced up to a 95 % reduction of liver ApoB mRNA and serum ApoB protein and a significant reduction of serum LDL in a mouse model with humanlike lipid profile [44]. Inhibition of ApoB synthesis with mipomersen (second-generation antisense oligonucleotide) decreased LDL cholesterol concentration by 25 % in 34 patients [45]. Another gene therapy product modulating cholesterol level is PCSK9, which binds to LDL receptors. A phase 1 trial demonstrated that inhibition of PCSK9 synthesis by RNA interference (RNAi) in 32 participants is a potentially effective mechanism to reduce LDL cholesterol [46]. Endothelial nitric oxide synthase (eNOS) gene transfer enhanced the antiatherogenic parameters of the atherosclerotic vessels and can reduce inflammatory cell infiltration and wall lipid deposition. Another way to decrease inflammation in atherogenic processes is targeting NF-kB which acts as a regulator of inflammatory events in endothelial cells [47].
15.5.3 Primary Systemic Hypertension
Hypertension has adverse effects on cardiovascular function and is a major risk factor for development of aortic dissection, intracerebral hemorrhage, ischemic heart disease, peripheral vascular disease, and renal insufficiency. The most effective antihypertensive drugs are short-term acting, must be taken everyday, and produce significant side effects. Finally, all of these medications decrease symptoms but do not cure the underlying causes; thus, their discontinuance results in the reappearance of high blood pressure (Chaps. 30 and 31). Genetic manipulation for hypertension in theory can induce a permanent effect with precise specificity based on molecular structure and, on a conceptual level, such an approach would be better than pharmacological therapy.
Potential gene strategies for control of hypertension are (i) use of antisense oligonucleotides to inhibit genes involved in elevated blood pressure pathways, like members of the renin-angiotensin system, angiotensin II, and beta-adrenergic receptors, and (ii) overexpression of genes encoding proteins that induce vasodilatation like kallikrein, atrial natriuretic peptide, or endothelial NOS.
15.5.3.1 Overexpression of Vasodilator Genes
DNA construct containing the human eNOS caused a significant reduction of systemic blood pressure for 6 weeks in hypertensive rats [48]. A single injection of the human adrenomedullin gene resulted in a prolonged reduction of blood pressure with a maximal reduction of 41 mmHg 9 days after gene delivery [49]. A maximal blood pressure reduction of 50 mmHg was observed in rats receiving kallikrein gene delivery, as compared to rats receiving only marker gene [50]. These and other studies provide support for the use of vasodilator gene overexpression to control hypertension.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


